2. 广西中医药大学海洋药物研究院,广西南宁 530200
2. Institute of Marine Drugs, Guangxi University of Chinese Medicine, Nanning, Guangxi, 530200, China
红树植物生长于热带和亚热带地区海岸潮间带,给红树微生物提供了特殊的宿主环境[1],致使红树微生物次级代谢产物的结构类型和生物活性具有多样性。近年来,从红树来源内生青霉中发现了许多结构新颖的活性次级代谢产物,如具有抗肿瘤活性的二氧哌嗪类生物碱penicisulfuranols A-C[2],具有抗虫活性的混源萜类化合物penicianstinoids C-E[3],具有α-葡萄糖苷酶抑制活性的倍半萜类化合物penicieudesmol F[4]、喹诺酮类生物碱(±)-oxypenicinolines A[5]和异香豆素类化合物(R)-2-chloro-3-(8-hydroxy-6-methoxy-1-oxo-1H-isochromen-3-yl)propyl acetate[6],以及具有抗炎活性的三烯类化合物pinophols C-D[7]等。以上研究表明从红树内生青霉中挖掘活性次级代谢产物作为药物的先导化合物具有很大潜力,是海洋天然活性产物的重要研究方向之一。本文对一株红树老鼠簕Acanthus ilicifolius L.来源的内生青霉Penicillium sp.GXIMD 03101的次级代谢产物进行研究,采用多种现代分离方法获取其中的化合物,并对化合物进行抗胰腺癌活性筛选,为进一步研究开发红树内生青霉来源的天然抗胰腺癌药物提供物质基础。
1 材料与方法 1.1 材料 1.1.1 主要仪器与试剂SHIMADZU LC-2030C高效液相色谱仪(日本岛津公司),Bruker AV-400 MHz超导核磁共振仪(瑞士Bruker公司),JNM-ECZ600R超导核磁共振仪(日本电子株式会社),EYELA N-1300D旋转蒸发仪(日本东京理化有限公司),普利塞斯EP225SM-DR电子天平(瑞士普利塞斯公司),WFH-2038暗箱式紫外分析仪(杭州齐威仪器有限公司),300-400目正相硅胶、HSGF254薄层层析硅胶板(烟台江友硅胶开发有限公司),十八烷基硅烷键合硅胶(上海麦克林生化科技股份有限公司),Sephadex LH-20 (上海麦克林生化科技股份有限公司),石油醚、乙酸乙酯、甲醇(分析纯,上海泰坦科技股份有限公司),甲醇(色谱纯,上海星可高纯溶剂有限公司)。
1.1.2 菌株来源内生青霉Penicillium sp.GXIMD 03101分离自红树植物老鼠簕Acanthus ilicifolius L.的根部,老鼠簕根样品于2020年7月采自广西山口红树林生态自然保护区。菌种存放于广西中医药大学海洋药物研究院,为青霉属Penicillium sp.菌株, GenBank号为MZ971181。
1.2 方法 1.2.1 菌种的发酵培养在超净台内用灭菌后的接种环从接有Penicillium sp.GXIMD 03101单一菌落的马铃薯葡萄糖琼脂(PDA) 培养基中挑取菌株,转接至3瓶已灭菌的马铃薯葡萄糖水(PDB) 液体培养基中。将PDB液体培养基置于37℃、180 r/min的摇床中振荡培养3 d获得种子液。将4 mL种子液转接至装有已灭菌80 g大米培养基的1 L三角瓶中,共150瓶,室温条件下培养30 d。
1.2.2 化合物提取与分离用40 L的乙酸乙酯萃取发酵后的12 kg大米培养基,重复萃取3次,每次间隔5 d,合并浓缩萃取液,得到浸膏150 g。采用硅胶柱层析、凝胶柱层析及半制备高效液相色谱对浸膏进行分离纯化,并利用核磁共振方法鉴定化合物的结构。
1.2.3 抗胰腺癌细胞活性测定方法由于其他化合物的质量相对比较少,且纯度不足,因此参考文献[8]的方法对部分化合物进行抗胰腺癌细胞活性测定。选用胰腺癌细胞(SW1990)为试验对象,阳性对照为氟尿嘧啶。将对数生长期的肿瘤细胞加到96孔细胞培养板(每孔200 μL),置于37℃培养箱中,在5% CO2的条件下培养24 h。每孔分别加入10 μL稀释的、不同浓度(5个梯度)的单体化合物,并以正常细胞为空白对照,培养48 h。每孔再加入10 μL 3-(4, 5-二甲基噻唑-2)-2, 5-二苯基四氮唑溴盐(MTT) 溶液,继续培养4 h。离心,去掉上清液,每孔加入100 μL二甲基亚砜(DMSO),振荡15 min,使晶体完全溶解。用酶标仪测定每孔在570 nm处的吸光度(OD值),计算抑制率(IR),IR(%)=[(OD对照-OD样品)/OD对照]×100%。
2 结果与分析 2.1 化合物的提取与分离结果40 L的乙酸乙酯萃取大米发酵产物后所得的浸膏经过减压正相硅胶柱层析(石油醚∶乙酸乙酯∶甲醇=100∶0∶0-0∶100∶0-0∶0∶100)得到10个组分(Fr.1-Fr.10)。
Fr.5 (8 g) 经过常压正相硅胶柱层析(石油醚∶乙酸乙酯∶甲醇=100∶0∶0-0∶100∶0-0∶0∶100) 得到9个组分(Fr.5-1-Fr.5-9),其中Fr.5-3为化合物11 (40.6 mg)。Fr.5-2 (50 mg) 经过Sephadex LH-20凝胶柱层析(纯甲醇)、半制备高效液相色谱(甲醇∶水=90∶10) 纯化得到化合物5 (2.0 mg,9.7 min)。Fr.5-4 (100 mg) 经过中压反相硅胶柱层析(甲醇∶水=10∶90-100∶0)、半制备高效液相色谱(甲醇∶水=90∶10) 纯化得到化合物4 (7.7 mg,14.0 min)。Fr.5-6经过半制备高效液相色谱(甲醇∶水=95∶5) 纯化得到化合物10 (4.6 mg,5.6 min)、化合物12 (5.1 mg,12.8 min) 和化合物13 (4.8 mg,15.7 min)。Fr.5-8 (4 g) 经过中压正相硅胶柱层析(石油醚∶乙酸乙酯∶甲醇=50∶50∶0-0∶100∶0-0∶0∶100)、中压反相硅胶柱层析(甲醇∶水=10∶90-100∶0)、半制备高效液相色谱(甲醇∶水=70∶30) 纯化得到化合物6 (3.6 mg,10.1 min)。
Fr.8 (6 g) 经过常压正相硅胶柱层析(石油醚∶乙酸乙酯∶甲醇=50∶50∶0-0∶100∶0-0∶0∶100) 得到7个组分(Fr.8-1-Fr.8-7)。Fr.8-3 (2 g) 经过中压反相硅胶柱层析(甲醇∶水=10∶90-100∶0) 得到9个组分(Fr.8-3-1-Fr.8-3-9)。Fr.8-3-5经过半制备高效液相色谱(甲醇∶水=65∶35) 纯化得到化合物2 (31.2 mg,21.7 min)。Fr.8-3-7经过半制备高效液相色谱(甲醇∶水=83∶17) 纯化得到化合物1 (37.8 mg,23.6 min)。Fr.8-4 (1.5 g) 经过中压反相硅胶柱层析(甲醇∶水=10∶90-100∶0)、半制备高效液相色谱(甲醇∶水=15∶85) 纯化得到化合物9 (13.5 mg,14.0 min)。Fr.8-6 (1 g) 经过中压反相硅胶柱层析(甲醇∶水=10∶90-100∶0)、半制备高效液相色谱(甲醇∶水=5∶95) 纯化得到化合物7 (9.2 mg,9.0 min) 和化合物8 (8.8 mg,17.0 min)。
Fr.10 (2 g) 经过中压正相硅胶柱层析(二氯甲烷∶甲醇=100∶0-0∶100) 得到8个组分(Fr.10-1-Fr.10-8)。Fr.10-2经过半制备高效液相色谱(甲醇∶水=90∶10) 纯化得到化合物3 (3.6 mg,17.7 min)。
2.2 化合物的结构从红树老鼠簕内生青霉Penicillium sp.GXIMD 03101中分离出13个化合物,它们的结构如图 1所示。
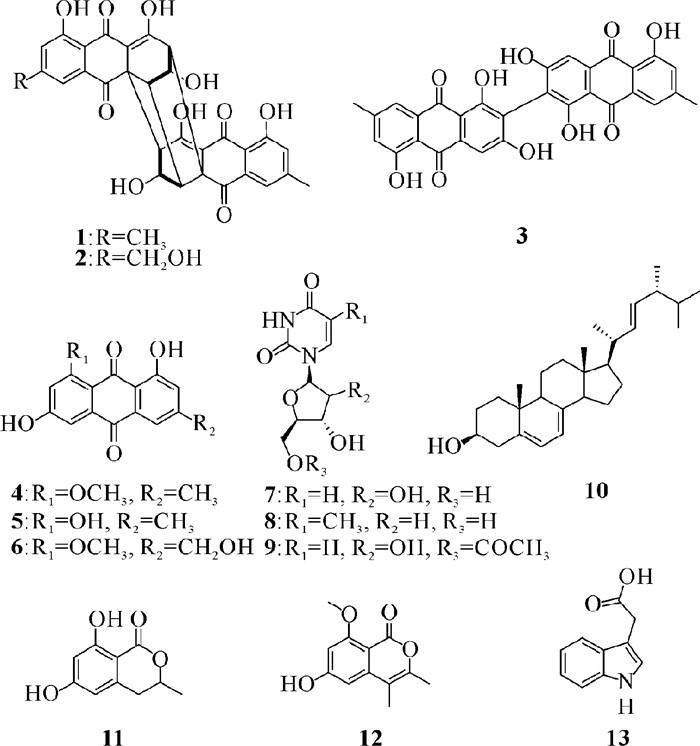
|
| 图 1 化合物1 - 13的化学结构 Fig. 1 Chemical structures of compounds 1 - 13 |
2.3 化合物的波谱数据
化合物1:黄色粉末,熔点为293℃,1H NMR (400 MHz,DMSO-d6,J in Hz) δH: 11.45 (s, 5-OH),7.46 (s, H-8),7.19 (s, H-6),5.45 (d, J=4.0 Hz, 2-OH),4.39 (s, H-2),3.37 (s, H-1),2.78 (d, J=4.0 Hz, H-3),2.42 (s, H-11);13C NMR (100 MHz,DMSO-d6)δC: 194.0 (C, C-9),185.9 (C, C-10),180.6 (C, C-4),160.1 (C, C-5),147.5 (C, C-7),132.0 (C, C-8a),124.0 (CH, C-6),120.4 (CH, C-8),114.2 (C, C-10a),106.1 (C, C-4a),68.5 (CH, C-2),58.3 (CH, C-3),55.6 (C, C-9a),47.7 (CH, C-1),21.4 (CH3, C-11)。将以上数据与文献[9]数据进行对比,确定化合物1为(+)-rugulosin A。
化合物2:黄色粉末,熔点为292℃,1H NMR (400 MHz,DMSO-d6,J in Hz) δH: 14.78 (br s, 4-OH),11.52 (d, J=12.0 Hz, 5, 5′-OH),7.59 (s, H-8),7.46 (s, H-8′),7.29 (s, H-6),7.19 (s, H-6, 6′),5.50 (d, J=36.0 Hz, 2, 2′-OH),4.62 (s, H-11),4.40 (s, H-2, 2′),3.38 (s, H-1, 1′),2.78 (t, J=4.0 Hz, H-3, 3′),2.43 (s, H-11′);13C NMR (100 MHz,DMSO-d6)δC: 194.1 (C, C-9),194.0 (C, C-9′),186.7 (C, C-4),185.9 (C, C-4′),180.6 (C, C-10),179.9 (C, C-10′),160.2 (C, C-5, 5′),152.0 (C, C-7),147.5 (C, C-7′),132.0 (C, C-8a, 8′a),124.0 (CH, C-6′),120.7 (CH, C-6),120.4 (CH, C-8′),117.0 (CH, C-8),114.9 (C, C-10a),114.2 (C, C-10′a),106.1 (C, C-4a),106.1 (C, C-4′a),68.5 (CH, C-2, 2′),62.0 (CH2, C-11),58.5 (CH, C-3),58.4 (CH, C-3′),55.6 (C, C-9a),55.6 (C, C-9′a),47.8 (CH, C-1, 1′),21.4 (CH3, C-11′)。将以上数据与文献[10]数据进行对比,确定化合物2为(+)-rugulosin B。
化合物3:橙红色粉末,熔点为270℃,1H NMR (600 MHz,DMSO-d6,J in Hz) δH: 12.78 (s, 1-OH),12.04 (s, 5-OH),7.27 (s, H-8),7.14 (s, H-6),6.69 (s, H-4),2.32 (s, H-11);13C NMR (150 MHz,DMSO-d6)δC: 189.4 (C, C-10),182.0 (C, C-9),164.6 (C, C-3),164.3 (C, C-1),161.0 (C, C-5),148.1 (C, C-7),133.2 (C, C-8a),131.2 (C, C-4a),123.7 (C, C-2),123.5 (CH, C-6),120.4 (CH, C-8),113.1 (C, C-10a),108.7 (C, C-9a),107.1 (CH, C-4),21.4 (CH3, C-11)。将以上数据与文献[11]数据进行对比,确定化合物3为1, 1′, 3, 3′, 5, 5′-hexahydroxy-7, 7′-dimethyl[2, 2′-bianthracene]-9, 9′, 10, 10′-tetraone。
化合物4:橙红色粉末,熔点为203℃,1H NMR (400 MHz,DMSO-d6,J in Hz) δH: 7.40 (d, J=1.2 Hz, H-4),7.16 (d, J=2.0 Hz, H-5),7.10 (s, H-2),6.79 (d, J=2.0 Hz, H-7),3.88 (s, H-12),2.37 (s, H-11);13C NMR (100 MHz,DMSO-d6)δC: 186.0 (C, C-9),182.4 (C, C-10),165.3 (C, C-8),163.5 (C, C-1),161.7 (C, C-6),146.4 (C, C-3),136.7 (C, C-4a),132.0 (C, C-10a),124.1 (CH, C-2),119.0 (CH, C-4),114.4 (C, C-9a),112.1 (C, C-8a),107.4 (CH, C-5),105.0 (CH, C-7),56.2 (CH3, C-12),21.3 (CH3, C-11)。将以上数据与文献[12]数据进行对比,确定化合物4为questin。
化合物5:橙红色粉末,熔点为255℃,1H NMR (600 MHz,DMSO-d6,J in Hz) δH: 7.52 (s, H-4),7.19 (s, H-2),7.12 (s, H-5),6.58 (s, H-7),2.42 (s, H-11);13C NMR (150 MHz,DMSO-d6)δC: 189.6 (C, C-9),181.5 (C, C-10),164.5 (C, C-8),166.0 (C, C-6),161.4 (C, C-1),148.1 (C, C-3),135.1 (C, C-10a),132.9 (C, C-4a),124.1 (CH, C-2),120.4 (CH, C-4),113.6 (C, C-9a),108.9 (C, C-8a),108.6 (CH, C-5),107.9 (CH, C-7),21.5 (CH3, C-11)。将以上数据与文献[13]数据进行对比,确定化合物5为emodin。
化合物6:黄色粉末,熔点为200℃,1H NMR (400 MHz,DMSO-d6,J in Hz)δH: 7.55 (d, J=0.8 Hz, H-4),7.17 (d, J=0.8 Hz, H-2),6.65 (d, J=1.6 Hz, H-5),4.56 (s, H-11),3.86 (s, H-12);13C NMR (100 MHz,DMSO-d6)δC: 185.3 (C, C-9),182.5 (C, C-10),164.5 (C, C-6),163.9 (C, C-8),161.8 (C, C-1),150.6 (C, C-3),136.7(C, C-4a),132.2(C, C-10a),120.8 (CH, C-2),115.5 (CH, C-4),115.4 (C, C-9a),112.7 (C, C-8a),108.5 (CH, C-5),105.2 (CH, C-7),62.1 (CH3, C-11),56.0 (CH3, C-12)。将以上数据与文献[12]数据进行对比,确定化合物6为questinol。
化合物7:无色粉末,熔点为165℃,1H NMR (400 MHz,CD3OD,J in Hz)δH: 8.01 (d, J=8.0 Hz, H-6),5.90 (d, J=4.0 Hz, H-1′),5.70 (d, J=8.0 Hz, H-5),4.16 (m, H-2′, 3′), 4.00 (m, H-4′),3.83 (d, J=2.8, 12.4 Hz, H-5′b),3.73 (d, J=3.2, 12.4 Hz, H-5′a);13C NMR (100 MHz,CD3OD)δC: 166.1 (C, C-4),152.4 (C, C-2),142.7 (CH, C-6),102.6 (CH, C-5),90.7 (CH, C-1′),86.3 (CH, C-4′),75.7 (CH, C-3′),71.3 (CH, C-2′),62.2 (CH2, C-5′)。将以上数据与文献[14]数据进行对比,确定化合物7为uridine。
化合物8:无色晶体,熔点为192℃,1H NMR (400 MHz,CD3OD,J in Hz)δH: 7.81 (d, J=1.2 Hz, H-6),6.27 (t, J=6.8 Hz, H-1′),4.39 (m, H-3′),3.90 (dd, J=3.2, 6.8 Hz, H-4′),3.75 (m, H-5′),2.22 (m, H-2′),1.87(d, J=1.2 Hz, H-7);13C NMR (100 MHz,CD3OD)δC: 166.4 (C, C-4),152.2 (C, C-2),138.1 (CH, C-6),111.5 (C, C-5),86.2 (CH, C-1′),88.8 (CH, C-4′),72.2 (CH, C-3′),41.1 (CH2, C-2′),62.2 (CH2, C-5′),12.4 (CH3, C-7)。将以上数据与文献[15]数据进行对比,确定化合物8为2′-deoxythymidine。
化合物9:无色粉末,熔点为202℃,1H NMR (400 MHz,DMSO-d6,J in Hz)δH: 7.62 (d, J=8.0 Hz, H-6),5.74 (d, J=4.8 Hz, H-1′),5.67 (d, J=8.0 Hz, H-5),4.23 (dd, J=3.2, 12 Hz, H-5′b),4.15 (dd, J=5.6, 12 Hz, H-5′a),4.07 (t, J=4.8 Hz, H-4′),3.98 (m, H-2′),3.93 (t, J=5.2 Hz, H-3′);13C NMR (100 MHz,DMSO-d6)δC: 170.3 (C, C-1″),163.1 (C, C-4),150.6 (C, C-2),140.8 (CH, C-6),102.1 (CH, C-5),88.7 (CH, C-1′),81.1 (CH, C-4′),72.7 (CH, C-2′),69.8 (CH, C-3′),63.8 (CH2, C-5′),20.1 (CH3, C-2″)。将以上数据与文献[16]数据进行对比,确定化合物9为5′-O-acetyl uridine。
化合物10:白色粉末,熔点为156℃,1H NMR (600 MHz,CDCl3,J in Hz)δH: 5.51 (m, H-6),5.33 (m, H-7),5.26 (m, H-23),5.18 (m, H-22),3.57 (m, H-3),2.40 (m, H-4a),2.21 (m, H-4b),1.18 (s, H-21),0.94 (s, H-19),0.92 (s, H-28),0.84 (s, H-27),0.83 (s, H-26),0.62 (s, H-18);13C NMR (150 MHz,CDCl3)δC: 141.4 (C, C-8),139.7 (C, C-5),135.6 (CH, C-22),132.0 (CH, C-23),119.7 (CH, C-6),116.4 (CH, C-7),70.5 (CH, C-3),55.8 (CH, C-17),54.6 (CH, C-14),46.3 (CH, C-9),42.8 (CH, C-24),42.8 (C, C-13),40.7 (CH2, C-4),40.5 (CH, C-20),39.1 (CH2, C-12),38.4 (CH2, C-1),37.1 (C, C-10),33.1 (CH, C-25),31.9 (CH2, C-2),28.3 (CH2, C-16),23.1 (CH2, C-15),21.2 (CH2, C-11),21.2 (CH3, C-21),20.0 (CH3, C-27),19.7 (CH3, C-26),17.7 (CH3, C-28),16.3 (CH3, C-19),12.1 (CH3, C-18)。将以上数据与文献[17]数据进行对比,确定化合物10为ergosta-5, 7, 22-triene-3β-ol。
化合物11:黄色油状,1H NMR (600 MHz,DMSO-d6,J in Hz)δH: 11.12 (s, 8-OH),10.63 (br s, 6-OH),6.23 (d, J=1.2 Hz, H-5),6.18 (d, J=2.4 Hz, H-7),4.68 (m, H-3),2.91 (dd, J=3, 0, 16.8 Hz, H-4a),2.80 (dd, J=11.4, 16.8 Hz, H-4b),1.38 (d, J=6.6 Hz, H-11);13C NMR (150 MHz,DMSO-d6)δC: 169.4 (C, C-1),164.4 (C, C-6),163.4 (C, C-8),142.2 (C, C-10),107.8 (CH, C-5),100.1 (CH, C-7),100.8 (C, C-9),75.3 (CH, C-3),33.7 (CH2, C-4),20.3 (CH3, C-11)。将以上数据与文献[18]数据进行对比,确定化合物11为(3R)-6-hydroxymellein。
化合物12:黄色油状,1H NMR (400 MHz,DMSO-d6,J in Hz)δH: 6.44 (d, J=1.6 Hz, H-7),6.38 (d, J=2.0 Hz, H-5),3.79 (s, H-11),2.18 (s, H-9),1.98 (s, H-10);13C NMR (100 MHz,DMSO-d6)δC: 164.4 (C, C-6),163.4 (C, C-8),157.5 (C, C-1),150.2 (C, C-3),142.5 (C, C-4a),106.2 (C, C-4),100.8 (C, C-8a),100.3 (CH, C-5),98.6 (CH, C-7),55.7 (CH3, C-11),17.0 (CH3, C-9),12.4 (CH3, C-10)。将以上数据与文献[19]数据进行对比,确定化合物12为6-hydroxy-8-methoxy-3, 4-dimethylisocoumarin。
化合物13:白色粉末,熔点为167℃,1H NMR (600 MHz,DMSO-d6,J in Hz)δH: 10.89 (s, H-1),7.49 (d, J=7.8 Hz, H-4),7.34 (d, J=8.4 Hz, H-7),7.22 (d, J=1.8 Hz, H-2),7.08 (t, J=7.8 Hz, H-6),6.97 (t, J=7.2 Hz, H-5),3.62 (s, H-10);13C NMR (150 MHz,DMSO-d6)δC: 174.5 (C, C-11),137.4 (C, C-8),128.5 (C, C-9),125.2 (CH, C-2),122.2 (CH, C-6),119.8 (CH, C-5),119.6 (CH, C-4),112.6 (CH, C-7),109.0 (C, C-3),32.4 (CH2, C-10)。将以上数据与文献[20]数据进行对比,确定化合物13为indole-3-acetic acid。
2.4 抗胰腺癌细胞活性对化合物1- 4进行抗胰腺癌细胞活性测定。如表 1所示,化合物1对胰腺癌细胞显示出较好的细胞毒活性,浓度为10 μmol/L时,对胰腺癌细胞的抑制率为75.6%。不同浓度下化合物2- 4对胰腺癌细胞的抑制率为1.3%-56.0%。阳性对照氟尿嘧啶浓度为5 μmol/L时,对胰腺癌细胞的抑制率为78.4%。
| 化合物浓度(μmol/L) Concentration of compound (μmol/L) | 抑制率(%) Inhibition ratio (%) | ||||
| 化合物1 Compound 1 | 化合物2 Compound 2 | 化合物3 Compound 3 | 化合物4 Compound 4 | 氟尿嘧啶Fluorouracil | |
| 2.5 | 71.4 | NA | NA | NA | - |
| 5.0 | 73.9 | NA | NA | 14.2 | 78.4 |
| 10.0 | 75.6 | 26.6 | NA | 17.7 | - |
| 20.0 | 74.4 | 36.4 | NA | 35.8 | - |
| 40.0 | 73.7 | 35.6 | 14.1 | 56.0 | - |
| Note: "NA" means inhibitive activity less than 10%;"-" means inhibitive activity less than 78% | |||||
3 结论
本文对一株红树老鼠簕来源的内生真菌Penicillium sp.GXIMD 03101的次级代谢产物进行研究,采用多种现代分离方法获得了13个化合物,经鉴定分别为6个蒽醌类化合物(1 - 6),3个核苷类化合物(7 - 9),1个甾醇类化合物(10),2个异香豆素类化合物(11,12),以及1个生物碱类化合物(13)。其中,化合物1对胰腺癌细胞显示出较好的细胞毒活性,浓度为10 μmol/L时对胰腺癌细胞的抑制率为75.6%。
| [1] |
曾尾女, 黄国雷, 王斌, 等. 红树来源青霉属真菌次级代谢产物与生物活性(2007-2020)[J]. 有机化学, 2021, 41(11): 4255-4278. |
| [2] |
ZHU M L, ZHANG X M, FENG H M, et al. Penicisulfuranols A-F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309[J]. Journal of Natural Products, 2017, 80(1): 71-75. DOI:10.1021/acs.jnatprod.6b00483 |
| [3] |
BAI M, ZHENG C J, CHEN G Y. Austins-type meroterpenoids from a mangrove-derived Penicillium sp.[J]. Journal of Natural Products, 2021, 84(8): 2104-2110. DOI:10.1021/acs.jnatprod.1c00050 |
| [4] |
CHEN H Q, QIU L M, WANG P, et al. Three new eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp.J-54[J]. Phytochemistry Letters, 2019, 33: 36-38. DOI:10.1016/j.phytol.2019.07.005 |
| [5] |
CHEN C M, CHEN W H, PANG X Y, et al. Pyrrolyl 4-quinolone alkaloids from the mangrove endophytic fungus Penicillium steckii SCSIO 41025:Chiral resolution, configurational assignment, and enzyme inhibitory activities[J]. Phytochemistry, 2021, 186: 112730. DOI:10.1016/j.phytochem.2021.112730 |
| [6] |
QIU P, CAI R L, LI L, et al. Three new isocoumarin derivatives from the mangrove endophytic fungus Penicillium sp.YYSJ-3[J]. Chinese Journal of Natural Medicines, 2020, 18(4): 256-260. DOI:10.1016/S1875-5364(20)30031-5 |
| [7] |
LUO Z W, TANG M M, ZHOU X M, et al. Five new triene derivatives from the fungus Penicillium herquei JX4[J]. Chemistry & Biodiversity, 2021, 18(5): e2100027. |
| [8] |
SUN L Y, WANG J, WANG Y F, et al. Cytotoxic and antiviral tetramic acid derivatives from the deep-sea-derived fungus Trichobotrys effuse DFFSCS021[J]. Tetrahedron, 2015, 71(49): 9328-9332. DOI:10.1016/j.tet.2015.10.010 |
| [9] |
AGUSTA A, OHASHI K, SHIBUYA H, et al. Bisan- thraquinone metabolites produced by the endophytic fungus Diaporthe sp.[J]. Chemical and Pharmaceutical Bulletin, 2006, 54(4): 579-582. DOI:10.1248/cpb.54.579 |
| [10] |
YAMAZAKI H, KOYAMA N, ŌMURA S, et al. New rugulosins, anti-MRSA antibiotics, produced by Penicillium radicum FKI-3765-2[J]. Organic Letters, 2010, 12(7): 1572-1575. DOI:10.1021/ol100298h |
| [11] |
CHEN T, YU C G, YANG B L. Structure elucidation and NMR assignments for two new quinones from fructus rhodomyrti of Rhodomyrtus tomentosa[J]. Chemistry of Natural Compounds, 2011, 47(4): 524-526. DOI:10.1007/s10600-011-9987-0 |
| [12] |
SAID G, HOU X M, LIU X, et al. Antimicrobial and cytotoxic activities of secondary metabolites from the soft coral derived fungus Aspergillus sp.[J]. Chemistry of Natural Compounds, 2019, 55(3): 531-533. DOI:10.1007/s10600-019-02732-5 |
| [13] |
LU Y H, WANG Z T, XU L S, et al. Three anthraquinones isolated from Aster tataricus L.f[J]. Journal of Chinese Pharmaceutical Sciences, 2003, 12(2): 112-113. |
| [14] |
MA Y T, QIAO L R, SHI W Q, et al. Metabolites produced by an endophyte Alternaria alternata isolated from Maytenus hookeri[J]. Chemistry of Natural Compounds, 2010, 46(3): 504-506. DOI:10.1007/s10600-010-9662-x |
| [15] |
吴旭东, 梅文莉, 邵长伦, 等. 中国南海蜂海绵Haliclona cymaeformis的化学成分研究[J]. 中国海洋药物杂志, 2011, 30(5): 12-17. |
| [16] |
JIA Y L, GUAN F F, MA J, et al. Pestalotiolide A, a new antiviral phthalide derivative from a soft coral-derived fungus Pestalotiopsis sp.[J]. Natural Product Sciences, 2015, 21(4): 227-230. DOI:10.20307/nps.2015.21.4.227 |
| [17] |
张起辉, 田黎, 闫政清, 等. 海洋真菌Nigrospora sphaerica中化学成分的研究[J]. 中国药学杂志, 2014, 49(1): 26-29. |
| [18] |
HAN N, YANG A M, MA S Y, et al. Chemical constituents and antioxidant and antibacterial activity of a fungal endophyte isolated from Plantago asiatica[J]. Chemistry of Natural Compounds, 2020, 56(5): 918-919. DOI:10.1007/s10600-020-03186-w |
| [19] |
CHEN S H, LIU Y Y, LIU Z M, et al. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities[J]. RSC Advances, 2016, 6(31): 26412-26420. DOI:10.1039/C6RA02566H |
| [20] |
ELSAYED Y, REFAAT J, ABDELMOHSEN U R, et al. Rhodozepinone, a new antitrypanosomal azepino-diindole alkaloid from the marine sponge-derived bacterium Rhodococcus sp.UA13[J]. Medicinal Chemistry Research, 2017, 26(11): 2751-2760. DOI:10.1007/s00044-017-1974-y |



