2. 中国科学院成都生物研究所,中国科学院环境与应用微生物重点实验室,四川省环境微生物重点实验室,四川成都 610041
2. CAS Key Laboratory of Environmental and Applied Microbiology, Environmental Microbiology Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan, 610041, China
急性淋巴细胞白血病(Acute Lymphoblastic Leukemia,ALL)是未成熟的B或T淋巴祖细胞在骨髓、外周血或中枢神经系统中异常聚集导致的恶性血液肿瘤,高发于儿童和青少年[1, 2]。近几十年来,新靶向药物和多药物化疗方案的联合使用极大地改善了ALL的临床治疗效果,5年无事件生存率(the five-year Event-Free Survival rate,5y-EFS)在85%以上[3]。然而,化疗药物严重的副作用及耐药复发严重制约了ALL的临床治疗效果,影响了ALL患儿预后。因此,急需开发和探索更多副作用小的抗白血病药物及新的治疗靶点[4]。
天然植物提取物,尤其以花青素为首的植物次生代谢产物,是世界上含量最丰富的水溶性黄酮类化合物,广泛存在于被子植物中[5]。迄今为止,花青素由于具有极强的抗氧化活性,引起全球范围内的高度关注。近期研究表明,花青素在结肠癌、乳腺癌、皮肤癌、肝癌、肺癌、食管癌及恶性血液肿瘤中具有潜在的抗肿瘤活性[6-9]。紫薯种植面广,产量高,花青素含量高,价格较蓝莓等高花青素作物更加低廉,是优质的花青素来源。因此,紫薯被认定为具有高营养价值和保健作用的功能性食品[10, 11],名列抗癌蔬菜榜首[12]。Guo等[13]报道紫薯花青素(Purple Sweet Potato Anthocyanin, PSPA)具有抗白血病活性,但紫薯花青素抗白血病的治疗靶点尚不清楚,仍需要进一步研究。
本研究采用分子对接技术,运用计算机预测紫薯花青素的潜在靶点,主要针对程序性细胞死亡(Programmed Cell Death,PCD),包括凋亡、焦亡、坏死性凋亡、铁死亡和铜死亡等相关细胞死亡过程的关键基因,以期预测出紫薯花青素最可能的分子靶点,并采用转录组测序和Western blot验证紫薯花青素对潜在靶点表达的影响,为花青素临床辅助治疗白血病提供数据支撑。
1 材料与方法 1.1 材料与试剂川紫4号紫薯及其相关信息由中国科学院成都生物研究所靳艳玲研究员提供。无水乙醇(分析纯)购自成都市科隆化学品有限公司。
1.2 方法 1.2.1 紫薯花青素的提取、纯化及鉴定紫薯块茎清水洗净,切薄片(2-5 mm),真空冷冻干燥后液氮研磨成粉末,100目过筛于-20℃保存备用。
花青素的提取、纯化及鉴定参考Hu等[14]的方法。首先,称取50.0 g紫薯粉末,用1.0 L含有0.5%盐酸的50%乙醇溶液充分混匀,静置24 h。然后,悬浊液4℃,4 000 r/min离心15 min;取上清液,40℃真空旋蒸仪(IKA RV10 Digital,Germany)浓缩;浓缩液上大孔树脂AB-8柱纯化并用乙酸乙酯分馏。最后,将水相部分冷冻干燥成干粉。以上实验在黑暗中操作。花青素提取物用液相质谱(AB SCIEX TRIPLE QUAD 4500, USA)分析鉴定。
1.2.2 分子对接分子对接参照Ibrahim等[15]的方法。受体蛋白3D结构下载自PubChem (https://www1.rcsb.org/),蛋白PDB ID(Bcl-2:1YSW;FDX1:3P1M;Parkin:4I1H;SLC7A11:7P9U)。花青素结构由ChemDraw 19.0及Chem3D软件绘制。分子对接结果图片由PyMOL软件(https://pymol.org/2/)生成。结合能 < -5.0 kcal/mol表示结合活性良好。
1.2.3 细胞培养条件NALM6人源B系急性淋巴白血病细胞购自上海生化与细胞生物研究所。NALM6细胞用含10%胎牛血清[Biological Industries(BI),以色列]、100 IU/mL青霉素和100 μg/mL链霉素(Gibco,美国)的1640培养液(Gibco,中国)培养。培养条件为37℃,5% CO2,90%湿度。
1.2.4 转录组测序急性淋巴白血病细胞NALM6用紫薯花青素处理24 h后,收集细胞,以未处理的NALM6细胞作为对照,每个处理2个生物学重复。RNA提取、建库、转录组测序及分析由北京诺禾致源科技股份有限公司完成。
1.2.5 Western blot用不同浓度(0 μg/mL、20 μg/mL、40 μg/mL、60 μg/mL)紫薯花青素处理NALM6细胞24 h,收集细胞,用预冷的PBS缓冲液(0.01 mol/L)清洗细胞2次,RIPA裂解液裂解细胞后超声破胞15 s,12 000 r/min离心10 min,收集上清液。以牛血清白蛋白为标准,Bradford染料结合测定法测定蛋白质浓度。30 μg蛋白上SDS-PAGE,然后将蛋白条带转移到PVDF膜上。随后依次孵育一抗、二抗,以GAPDH为对照,化学成像仪[e-BLOT Touch Imager,易孛特生命科学(上海)有限公司]检测蛋白条带。
2 结果与分析 2.1 紫薯花青素的成分从川紫4号紫薯中鉴定出25种花青素成分,主要包含矢车菊素及其衍生物、芍药色素及其衍生物、天竺葵色素及其衍生物(表 1),其结构见图 1。其中矢车菊素及其衍生物有10种,含量最高,占总花青素的53.80%;其次是芍药色素及其衍生物,有13种,含量占总花青素的45.92%;含量和种类最低的是天竺葵色素及其衍生物,仅有2种,其含量仅占总花青素的0.28%。
| 编号 No. |
花青素类物质 PSPAs compounds |
分子量 Molecular weight |
质谱碎片信息 Mass spectrometry fragment information |
相对含量(%) Relative content (%) |
| 1 | Cyanidin 3-sophoroside-5-glucoside | 773 | 611, 449, 287 | 1.99 |
| 2 | Peonidin 3-sophoroside-5-glucoside | 787 | 625, 463, 301 | 8.27 |
| 3 | Pelargonidin 3-sophoroside-5-glucoside | 757 | 595, 433, 271 | 0.12 |
| 4 | Cyanidin 3-p-hydroxybenzoyl sophoroside-5-glucoside | 893 | 731, 449, 287 | 1.28 |
| 5 | Peonidin 3-p-hydroxybenzoyl sophoroside-5-glucoside | 907 | 745, 463, 301 | 3.03 |
| 6 | Cyanidin 3-(6-caffeoyl sophoroside)-5-glucoside | 935 | 773, 449, 287 | 2.73 |
| 7 | Peonidin 3-caffeoyl sophoroside-5-glucoside | 949 | 787, 463, 301 | 3.84 |
| 8 | Cyanidin 3-(6-feruloyl sophoroside)-5-glucoside | 949 | 787, 449, 287 | 3.70 |
| 9 | Peonidin 3-(6-feruloyl sophoroside)-5-glucoside | 963 | 801, 463, 301 | 0.39 |
| 10 | Pelargonidin 3-feruloyl sophoroside-5-glucoside | 933 | 771, 433, 271 | 0.15 |
| 11 | Cyanidin 3-(6-coumaryl sophoroside)-5-glucoside | 919 | 757, 449, 287 | 0.18 |
| 12 | Peonidin 3-(6-coumaryl sophoroside)-5-glucoside | 933 | 771, 463, 301 | 0.20 |
| 13 | Cyanidin 3-(6″, 6`″-dicaffeoyl sophoroside)-5-glucoside | 1 097 | 935, 449, 287 | 0.98 |
| 14 | Peonidin 3-(6″, 6`″-dicaffeoyl sophoroside)-5-glucoside | 1 111 | 949, 463, 301 | 2.82 |
| 15 | Cyanidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | 1 055 | 893, 449, 287 | 14.17 |
| 16 | Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | 1 069 | 907, 463, 301 | 22.48 |
| 17 | Cyanidin 3-(6-caffeoyl-6-feruloyl sophoroside)-5-glucoside | 1 111 | 949, 449, 287 | 3.15 |
| 18 | Peonidin 3-(6-caffeoyl-6-feruloyl sophoroside)-5-glucoside | 1 125 | 963, 463, 301 | 3.19 |
| 19 | Peonidin 3-feruloyl-p-caffeoyl sophoroside-5-glucoside | 1 127 | 965, 463, 301 | 0.56 |
| 20 | Peonidin 3-feruloyl-p-coumaryl sophoroside-5-glucoside | 1 109 | 947, 463, 301 | 0.26 |
| 21 | Cyanidin 3-(6″, 6`″-diferuloyl sophoroside)-5-glucoside | 1 125 | 963, 449, 287 | 3.00 |
| 22 | Peonidin 3-(6″, 6`″-diferuloyl sophoroside)-5-glucoside | 1 139 | 977, 463, 301 | 0.02 |
| 23 | Peonidin 3-caffeoyl-p-coumaryl sophoroside-5-glucoside | 1 095 | 933, 463, 301 | 0.56 |
| 24 | Cyanidin 3-feruloyl-p-hydroxybenzoyl sophoroside-5-glucoside | 1 069 | 907, 449, 287 | 22.63 |
| 25 | Peonidin 3-feruloyl-p-hydroxybenzoyl sophoroside-5-glucoside | 1 083 | 921, 463, 301 | 0.31 |
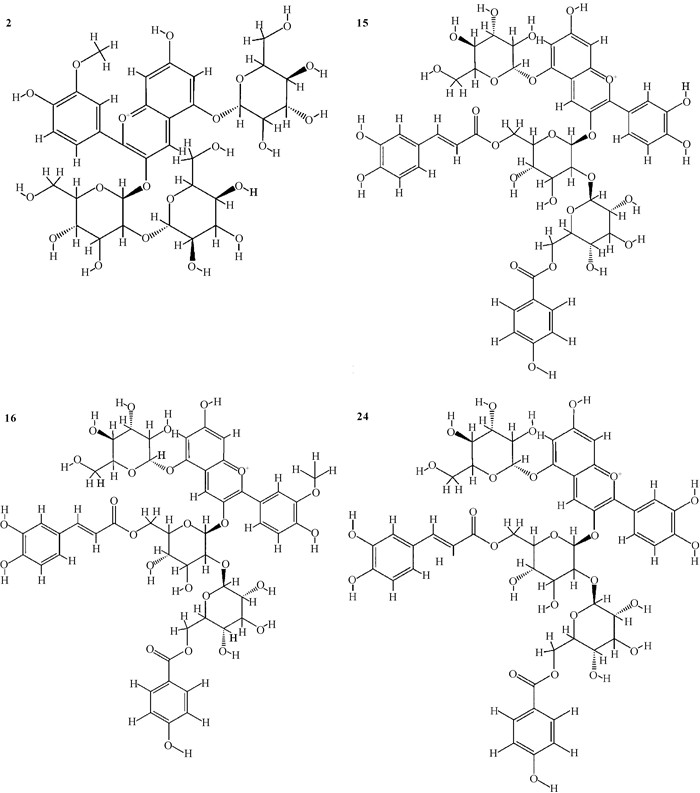
|
| 2 : Peonidin 3-sophoroside-5-glucoside, 15 : Cyanidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside, 16 : Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside, 24 : Cyanidin 3-feruloyl-p-hydroxybenzoyl sophoroside-5-glucoside 图 1 紫薯花青素主要成分结构 Fig. 1 Main component structure of purple sweet potato anthocyanins |
2.2 紫薯花青素与Bcl-2蛋白分子对接
Bcl-2家族蛋白是细胞凋亡的中心调控因子,是典型的抗凋亡蛋白,在多种肿瘤中过度表达,从而促进肿瘤的发生、进展和耐药。因此,抑制Bcl-2的表达能诱导凋亡。紫薯花青素与Bcl-2蛋白的相互作用见图 2。从图 2(a)可以看出,紫薯花青素可以结合到Bcl-2蛋白上,与受体上5个氨基酸残基发生氢键作用,最低结合能为-6.7 kcal/mol,说明紫薯花青素结合活性良好。
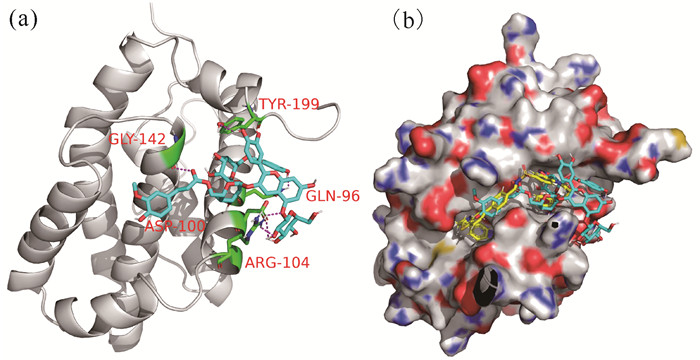
|
| (a) The binding sites between PSPA and Bcl-2;(b) The binding sites between Bcl-2 and both PSPA and ABT-737, Bcl-2 protein is white, PSPA molecule is shown in blue, active site interacting residues are shown in green, hydrogen bonds are indicated with magenta dashed lines, ABT-737 is shown in yellow 图 2 紫薯花青素与Bcl-2蛋白相互作用 Fig. 2 Interaction between Bcl-2 protein and PSPA |
2.3 紫薯花青素与Parkin蛋白分子对接
如图 3所示,紫薯花青素与Parkin蛋白有良好的结合活性,可与8个氨基酸残基发生相互作用,分别是LYS-412、THR-410、TRP-447、ARG-234、LYS-427、GLY-429、VAL-465以及ARG-256,其结合能最低达到-8.4 kcal/mol。
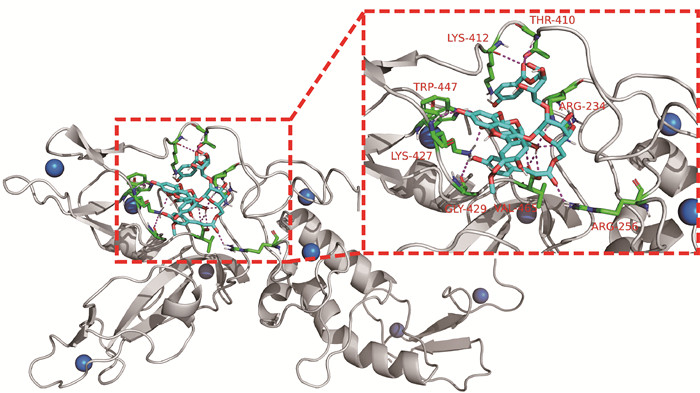
|
| Parkin protein is white, PSPA molecule is shown in blue, active site interacting residues are shown in green, hydrogen bonds are indicated with magenta dashed lines, Zn ions are shown as blue balls 图 3 紫薯花青素与Parkin蛋白相互作用 Fig. 3 Interaction between Parkin protein and PSPA |
2.4 紫薯花青素与SLC7A11蛋白分子对接
如图 4所示,紫薯花青素可以与5个氨基酸残基发生相互作用,其结合能最低达到-10.4 kcal/mol,绝大多数结合能都低于-9.0 kcal/mol。由此可见,紫薯花青素与SLC7A11的结合活性要优于Bcl-2和Parkin蛋白。
|
| SLC7A11 protein is white, PSPA molecule is shown in blue, active site interacting residues are shown in green, hydrogen bonds are indicated with magenta dashed lines 图 4 紫薯花青素与SLC7A11蛋白相互作用 Fig. 4 Interaction between SLC7A11 protein and PSPA |
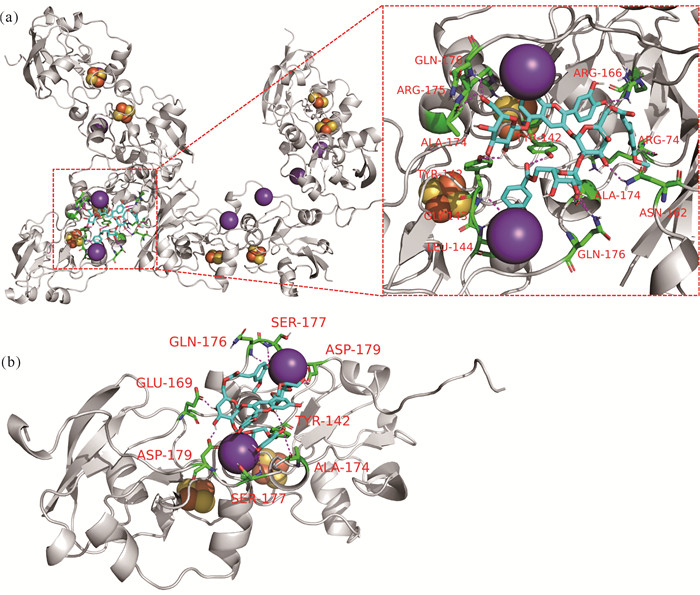
2.5 紫薯花青素与Fe-S簇蛋白和FDX1蛋白分子对接
将紫薯花青素分别与Fe-S簇蛋白和FDX1蛋白进行分子对接,结果表明紫薯花青素可以和两种蛋白结合。如图 5所示,紫薯花青素可以和Fe-S簇蛋白12个氨基酸残基发生相互作用,和FDX1蛋白8个氨基酸残基结合,其结合能分别为-9.6 kcal/mol和-7.5 kcal/mol,表明紫薯花青素与两个蛋白结合性能良好。
|
| (a) FDX1 and Fe-S clusters are white.(b) PSPA molecule is shown in blue, active site interacting residues are shown in green, hydrogen bonds are indicated with magenta dashed lines, K ions are shown as purple balls, Fe2-S2 cluster is shown as orange/yellow balls 图 5 紫薯花青素与FDX1和Fe-S簇蛋白相互作用 Fig. 5 Interaction between of FDX1, Fe-S clusters protein and PSPA |
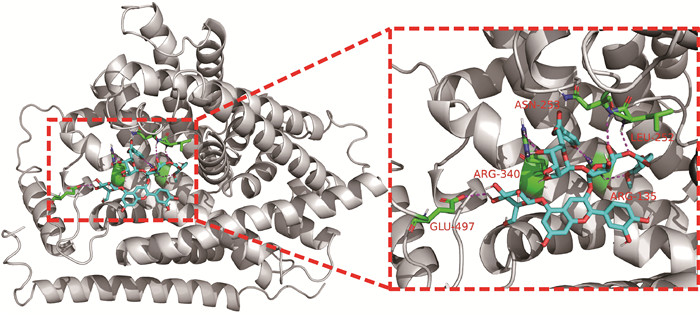
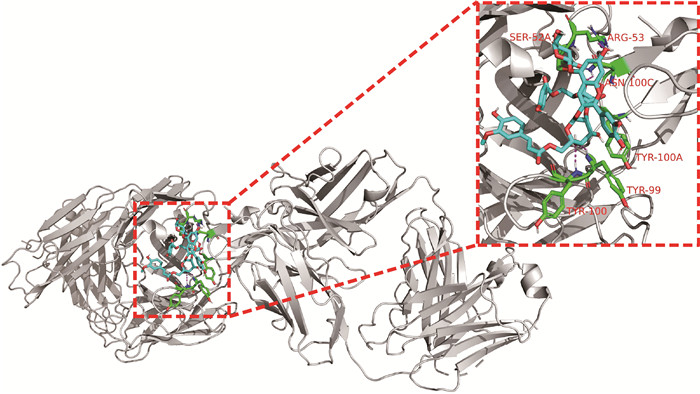
2.6 紫薯花青素与c-Myc蛋白分子对接
为了印证紫薯花青素分子对接结果与蛋白表达结果的一致性,还进行了紫薯花青素与癌蛋白c-Myc的分子对接分析,结果见图 6。花青素和c-Myc的6个氨基酸残基发生相互作用,其结合能最低为-8.5 kcal/mol,说明紫薯花青素可以结合c-Myc蛋白,抑制其表达。
|
| C-Myc is white, PSPA molecule is shown in blue, active site interacting residues are shown in green, hydrogen bonds are indicated with magenta dashed lines 图 6 紫薯花青素与c-Myc蛋白相互作用 Fig. 6 Interaction between c-Myc protein and PSPA |
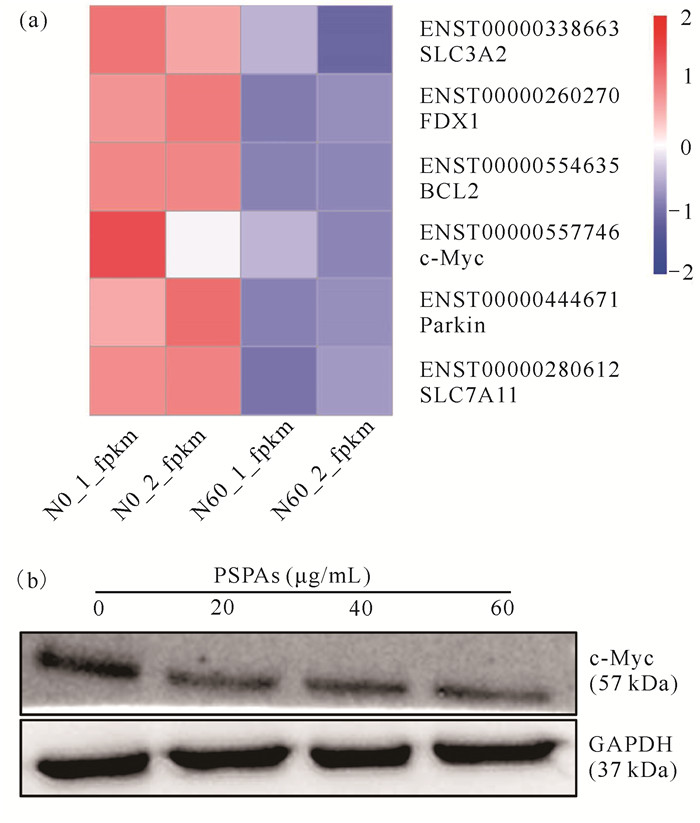
2.7 紫薯花青素对Bcl-2、Parkin、SLC7A11、FDX1及c-Myc蛋白表达的影响
转录组测序结果显示,经紫薯花青素处理24 h后,处理组中Bcl-2、Parkin、SLC7A11、FDX1及c-Myc的表达均显著低于对照组[图 7(a)]。Western blot检测c-Myc蛋白表达水平变化显示, 紫薯花青素能显著降低c-Myc蛋白表达水平[图 7(b)],进一步验证了分子对接结果。
|
| (a) NALM6 cells are treated with 20 μg/mL, 40 μg/mL or 60 μg/mL PSPAs for 24 h; (b) The levels of c-Myc are determined by Western blot analysis, GAPDH is used as the loading control 图 7 紫薯花青素处理后蛋白表达变化 Fig. 7 Changes of protein expression after PS PA treatment |
3 讨论
从表 1可以看出,紫薯花青素主要是酰化花青素,占总花青素的90%左右,与Lee等[16]的报道一致。含量最高的是24号花青素矢车菊素3-阿魏酸-对羟基苯甲酰槐糖苷-5-葡萄糖苷(Cyanidin 3-feruloyl-p-hydroxybenzoyl sophoroside-5-glucoside),达到22.63%。花青素主要是葡萄糖苷类化合物,在光照、热及pH改变条件下不稳定,易被氧化成对应的葡萄糖苷酸,而酰化反应可以显著增强花青素的稳定性[17]。另外,在矢车菊素、芍药色素及天竺葵色素3种花青素成分中,矢车菊素的抗氧化活性最强,已被报道在多种肿瘤中具有抗癌活性[18]。24号花青素是矢车菊素双乙酰化衍生物,其稳定性和抗氧化活性具备一定的优势,同时其含量最高,因此该成分被选择作为配体进行分子对接研究分析。
众所周知,诱导肿瘤细胞发生程序性细胞死亡是治疗恶性肿瘤的有效机制。因此,为研究紫薯花青素抗白血病的活性,对多种程序性细胞死亡的关键蛋白进行了分子对接分析。图 2结果已经表明紫薯花青素可以结合到抗凋亡蛋白Bcl-2上,进一步对比Bcl-2抑制剂ABT-737的结合位点显示[图 2(b)],紫薯花青素与Bcl-2结合位点和ABT-737结合位点有明显的重叠[19],说明紫薯花青素也能较好地抑制Bcl-2蛋白的表达,最终诱导细胞发生凋亡。前期研究也显示,紫薯花青素处理ALL细胞NALM6后,细胞凋亡率较未处理细胞提高了10%[13],在细胞生理水平上验证了分子对接的结果。
在坏死性凋亡和焦亡方面,Parkin蛋白是一种E3泛素连接酶,在泛素化级联反应的最后一环起重要作用。活化的Parkin蛋白可以泛素化RIPK3以及NLRP3,从而负调控坏死小体和炎症小体的合成[20, 21]。因此,抑制Parkin蛋白,可以促进坏死性凋亡和焦亡的发生[22]。紫薯花青素可以结合Parkin蛋白,Riley等[23]报道Parkin蛋白ARG-234、VAL-465、ARG-256残基是该蛋白的关键位点,在蛋白的活性和结构上起决定性的作用。因此紫薯花青素结合这些位点可以有效破坏Parkin蛋白的活性和结构稳定性,从而抑制蛋白的表达,导致细胞坏死小体和炎症小体的形成,促进坏死性凋亡和焦亡的发生。
胱氨酸转运蛋白SLC7A11是铁死亡通路的关键蛋白,抑制其表达可以在一定程度上诱导细胞发生铁死亡[24]。分子对接分析表明,紫薯花青素可以很好地结合到SLC7A11蛋白上。更重要的是,Parker等[25]研究表明ARG-135位点突变会直接导致SLC7A11蛋白失活。从图 5可以看出,紫薯花青素可以结合ARG-135位点,从而影响SLC7A11蛋白的结构和活性。Birsen等[26]研究表明药理化合物遗传性失活SLC7A11可以诱导白血病细胞发生铁死亡。由此可见,紫薯花青素也可以起到异曲同工的作用。
程序性细胞死亡方式除了上述几种,最近又有学者报道了一种不同于已知细胞死亡机制的新型细胞死亡方式——铜死亡[27]。铜死亡涉及一个关键蛋白——铁氧还蛋白1(FDX1)。FDX1是小分子抗癌药Elesclomol的靶蛋白,Elesclomol可以靶向作用于FDX1诱导细胞发生铜死亡。FDX1既能作用于Fe-S簇蛋白的生物合成,又能还原Cu2+生成Cu+,是铜死亡通路最关键的蛋白[28]。研究结果表明,紫薯花青素可以良好地结合在Fe-S簇蛋白和FDX1蛋白上,其结合位点均位于Fe-S离子簇及K+附近,与Elesclomol结合位点非常接近[28],据此推断紫薯花青素也可以抑制Fe-S簇蛋白和FDX1蛋白的表达,诱导细胞发生铜死亡。然而,铜死亡是程序性细胞死亡领域最前沿的研究成果,目前尚无铜死亡与白血病相关的研究。因此,分子对接的结果也为后续提供了新的视野,为白血病的临床治疗开拓了新的研究思路,是下一步研究的重点关注方向。
为进一步验证分子对接结果,本研究对紫薯花青素处理的ALL细胞NALM6进行了验证。Guo等[13]报道紫薯花青素可以抑制ALL细胞的活性。转录组测序结果再次验证紫薯花青素可以抑制Bcl-2、Parkin、SLC7A11、FDX1及c-Myc的表达,再加上Western blot实验结果进一步验证了紫薯花青素确实可以影响c-Myc蛋白的表达水平[图 7(b)],在转录组和蛋白水平验证了分子对接结果与生理生化结果高度一致。综上所述,分子对接结果可以初步预测紫薯花青素的潜在靶点。
本研究鉴定出25种花青素成分,为紫薯花青素应用于白血病临床治疗提供了基础信息,并基于细胞程序性死亡,分析了紫薯花青素潜在的抗白血病分子机制。从分子对接结果可以看出,紫薯花青素可能作用于凋亡、焦亡、坏死性凋亡、铁死亡及铜死亡等不同的方式,尤其是凋亡、铁死亡和铜死亡。首先,紫薯花青素与Bcl-2的结合位点与已知的Bcl-2抑制剂ABT-737的结合位点高度重合,表明紫薯花青素也可能是Bcl-2的抑制剂之一。其次,紫薯花青素除了能结合SLCA711外,前期研究结果还发现紫薯花青素可以诱导ALL细胞活性氧(Reactive Oxygen Species, ROS)生成[13],这些都是细胞发生铁死亡的典型特征。最后,铜死亡是最新提出的一种细胞死亡方式[27],其分子机制有待进一步详细研究,而分子对接的结果提示紫薯花青素具有铜死亡诱导剂的可能性,为紫薯花青素应用于临床提供新的研究思路。
4 结论本研究分析、纯化、鉴定出25种紫薯花青素,并得到了其相应的结构式。通过分子对接分析初步揭示了紫薯花青素治疗白血病的潜在靶蛋白。结果表明紫薯花青素能与凋亡、焦亡、坏死性凋亡、铜死亡以及铁死亡的关键蛋白结合,并在转录组水平和蛋白水平初步验证了分子对接的结果。本研究结果为后续分子生物学和细胞生物学实验提供了理论依据,也为白血病临床治疗提供了新的思路。
| [1] |
MARKE R, VAN LEEUWEN F N, SCHEIJEN B. The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia[J]. Haematologica, 2018, 103(4): 2-11. |
| [2] |
GAUDICHON J, JAKOBCZYK H, DEBAIZE L, et al. Mechanisms of extramedullary relapse in acute lymphoblastic leukemia: Reconciling biological concepts and clinical issues[J]. Blood Reviews, 2019, 36: 40-56. DOI:10.1016/j.blre.2019.04.003 |
| [3] |
OLIVAS-AGUIRRE M, POTTOSIN I, DOBROVINSKAYA O. Mitochondria as emerging targets for therapies against T cell acute lymphoblastic leukemia[J]. Journal of Leukocyte Biology, 2019, 105(5): 935-946. DOI:10.1002/JLB.5VMR0818-330RR |
| [4] |
ADAMSON P C. Improving the outcome for children with cancer: Development of targeted new agents[J]. CA-A Cancer Journal for Clinicians, 2015, 65(3): 212-220. DOI:10.3322/caac.21273 |
| [5] |
LIN B W, GONG C C, SONG H F, et al. Effects of anthocyanins on the prevention and treatment of cancer[J]. British Journal of Pharmacology, 2017, 174(11): 1226-1243. DOI:10.1111/bph.13627 |
| [6] |
ZHAO X, FENG P, HE W, et al. The prevention and inhibition effect of anthocyanins on colorectal cancer[J]. Current Pharmaceutical Design, 2019, 25(46): 4919-4927. |
| [7] |
SZYMANOWSKA U, BARANIAK B, BOGUCKAKOCKA A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from polana raspberry (Rubus idaeus L.) fruit and juice—In vitro study[J]. Molecules, 2018, 23(7): 1812. DOI:10.3390/molecules23071812 |
| [8] |
LEÓN-GONZÁLEZ A J, SHARIF T, AUGER C, et al. Anthocyanin-rich bilberry extract induces apoptosis in acute lymphoblastic leukemia cells via redox-sensitive epigenetic modifications[J]. Journal of Functional Foods, 2018, 44: 227-234. DOI:10.1016/j.jff.2018.03.013 |
| [9] |
DIACONEASA Z, STIRBU I, XIAO J, et al. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention[J]. Biomedicines, 2020, 8(9): 336. DOI:10.3390/biomedicines8090336 |
| [10] |
XU J, SU X, LIM S, et al. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40[J]. Food Chemistry, 2015, 186: 90-96. DOI:10.1016/j.foodchem.2014.08.123 |
| [11] |
KONCZAK-ISLAM I, YOSHIMOTO M, HOU D X, et al. Potential chemopreventive properties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L.)[J]. Journal of Agricultural and Food Chemistry, 2003, 51(20): 5916-5922. DOI:10.1021/jf030066o |
| [12] |
张鹏云. 紫薯的营养功效与保健[J]. 吉林蔬菜, 2011(5): 10. DOI:10.3969/j.issn.1672-0180.2011.05.009 |
| [13] |
GUO L, LIU J, YANG Y, et al. Purple sweet potato anthocyanins elicit calcium overload-induced cell death by inhibiting the calcium-binding protein S100A4 in acute lymphoblastic leukemia[J]. Food Bioscience, 2021, 42: 101214. DOI:10.1016/j.fbio.2021.101214 |
| [14] |
HU Y, DENG L, CHEN J, et al. An analytical pipeline to compare and characterise the anthocyanin antioxidant activities of purple sweet potato cultivars[J]. Food Chemistry, 2016, 194: 46-54. DOI:10.1016/j.foodchem.2015.07.133 |
| [15] |
IBRAHIM R S, EL-BANNA A A. Network pharmacology-based analysis for unraveling potential cancer-related molecular targets of Egyptian propolis phytoconstituents accompanied with molecular docking and in vitro studies[J]. Royal Society of Chemistry, 2021, 11: 11610-11626. |
| [16] |
LEE M J, PARK J S, CHOI D S, et al. Characterization and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in Korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS[J]. Journal of Agricultural and Food Chemistry, 2013, 61(12): 3148-3158. DOI:10.1021/jf3055455 |
| [17] |
LI J, LI X, ZHANG Y, et al. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices[J]. Food Chemistry, 2013, 136(3): 1429-1434. |
| [18] |
LI D, WANG P, LUO Y, et al. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade[J]. Critical Reviews in Food Science and Nutrition, 2017, 57(8): 1729-1741. DOI:10.1080/10408398.2015.1030064 |
| [19] |
OLTERSDORF T, ELMORE S W, SHOEMAKER A R, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours[J]. Nature, 2005, 435(7042): 677-681. DOI:10.1038/nature03579 |
| [20] |
MOUTON-LIGER F, ROSAZZA T, SEPULVEDADIAZ J, et al. Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop[J]. Glia, 2018, 66(8): 1736-1751. DOI:10.1002/glia.23337 |
| [21] |
LEE S B, KIM J J, HAN S-A, et al. The AMPK-Parkin axis negatively regulates necroptosis and tumorigenesis by inhibiting the necrosome[J]. Nature Cell Biology, 2019, 21(8): 940-951. DOI:10.1038/s41556-019-0356-8 |
| [22] |
李权, 权美玉, 张金三. Parkin调控凋亡、坏死性凋亡和焦亡的研究进展[J]. 中国细胞生物学学报, 2021, 43(12): 2433-2440. |
| [23] |
RILEY B E, LOUGHEED J C, CALLAWAY K, et al. Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases[J]. Nature Communications, 2013, 4: 1982. DOI:10.1038/ncomms2982 |
| [24] |
KOPPULA P, ZHUANG L, GAN B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy[J]. Protein & Cell, 2021, 12(8): 599-620. |
| [25] |
PARKER J L, DEME J C, KOLOKOURIS D, et al. Molecular basis for redox control by the human cystine/glutamate antiporter system xc(-)[J]. Nature Communications, 2021, 12(1): 7147. DOI:10.1038/s41467-021-27414-1 |
| [26] |
BIRSEN R, LARRUE C, DECROOCQ J, et al. APR-246 induces early cell death by ferroptosis in acute myeloid leukemia[J]. Haematologica, 2022, 107(2): 403-416. |
| [27] |
TSVETKOV P, COY S, PETROVA B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins[J]. Science, 2022, 375(6586): 1254-1261. DOI:10.1126/science.abf0529 |
| [28] |
TSVETKOV P, DETAPPE A, CAI K, et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress[J]. Nature Chemical Biology, 2019, 15(7): 681-689. DOI:10.1038/s41589-019-0291-9 |



