2. 广西医科大学第一附属医院学科建设办公室,广西南宁 530021
2. Disciplines Construction Office, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, China
细菌直接暴露在变幻莫测的环境中,经常会遇到比较恶劣的环境条件,要想在逆境中生存,就必须能够及时感应环境的变化,并且对其细胞生理生化过程作出适当、准确的调控。细菌生活的环境中,营养物质匮乏对细菌的生存是一个关键因子,当营养物质匮乏时,为了“紧缩开支”,度过危机,细菌会产生应急反应。细菌的应急反应是一个全局调控过程,受细胞内的两种小分子物质鸟苷四磷酸(ppGpp)和鸟苷五磷酸(pppGpp)调控,这两种物质统称为(p)ppGpp[1-3]。(p)ppGpp在细菌的应急反应过程中起全局调控作用,与细菌致病因子的合成和分泌有关,能够调控细菌许多基因的复制、转录以及表达[4],至今仍是生物学研究热点之一。
植物病原细菌发生应急反应后,相关基因会发生突变,导致细菌的致病力下降,对宿主细胞的侵染力和繁殖能力也明显降低[5-11]。目前关于植物病原细菌与应急反应相关的研究较少,仅在解淀粉欧文氏菌(Erwinia amylovora)[5]、丁香假单胞杆菌(Pseudomonas syringae)[6, 7]、黑腐果胶杆菌(Pectobacterium atrosepticum)[8]、柑橘黄单胞菌柑橘亚种(Xanthomonas citri subsp.citri)[9]、十字花科黑腐病菌(X.campestris pv.campestris,Xcc)[10, 11]有相关报道。本文主要综述了上述病原细菌的应急反应研究进展,旨在使研究者对植物病原细菌的应急反应和致病机制有更全面、更深入的认识,为寻找防治细菌性病害的药物靶点,从而有效地防治植物细菌病害提供理论基础。
1 应急反应概述革兰氏阴性细菌中胞内(p)ppGpp的积累主要由RelA和SpoT这两种酶决定,这两种酶分别是基因relA和基因spoT的编码产物。RelA具有(p)ppGpp合成酶活性,并且只在氨基酸饥饿时才合成(p)ppGpp。基因spoT编码一种双功能酶SpoT,具有较强的(p)ppGpp水解酶活性和较弱的(p)ppGpp合成酶活性[12, 13]。细菌的应急反应不仅在氨基酸饥饿时才会被诱导发生,还有许多其他的应急情况,如在碳源、氮源、能量、磷酸、铁离子或者脂肪酸匮乏、氧化应激时,革兰氏阴性细菌也会利用SpoT合成(p)ppGpp,度过恶劣的环境[14-16],并且在危机过后,SpoT会立即水解胞内高浓度的(p)ppGpp,使细菌胞内(p)ppGpp含量保持平衡。
革兰氏阳性细菌基因组中一般只含有一个基因rel,它编码一个双功能酶Rel,具有合成和水解(p)ppGpp的活性[17]。在枯草芽孢杆菌(Bacillus subtilis)中还鉴定出另外两个基因,分别为yjbM和ywaC,它们编码的酶是只具有合成(p)ppGpp功能的小分子蛋白质[18, 19]。在链球菌(Streptococcus)中也鉴定出两个只具有合成(p)ppGpp活性的蛋白质,分别为RelP和RelQ[20]。近年来在植物中也发现了许多RelA/SpoT同源物质,这些物质被称为Rsh[21-25]。
目前,在许多革兰氏阴性病原菌中已证实relA和spoT参与细菌的应急反应。(p)ppGpp可与转录因子DksA直接结合,降低RNA聚合酶(RNA polymerase,RNAP)与相关基因复合物的稳定性[26]。(p)ppGpp也可以间接调节RNAP核心酶与不同σ因子的亲和力,调控不同基因的表达[27]。(p)ppGpp在应急反应中与病原菌的多种生理生化过程相关[28](图 1),包括DNA的转录、复制、翻译,病原菌的致病力、细菌的繁殖、运动以及潜伏等[6, 9, 15, 26, 29]。
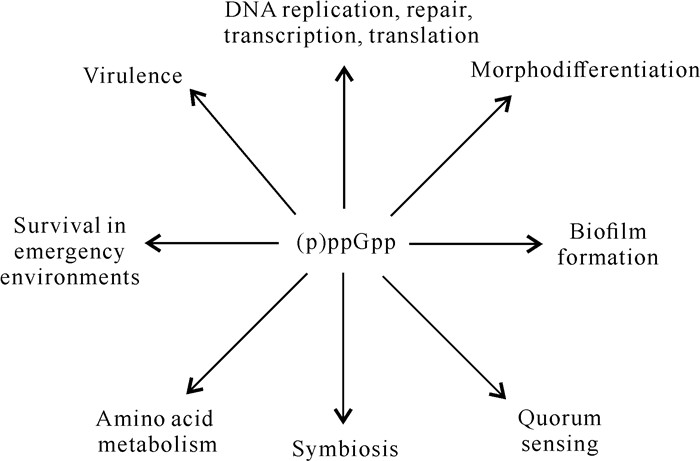
|
| 图 1 (p) ppGpp调控细胞过程的示意图 Fig. 1 Schematic diagram of (p)ppGpp regulating cellular processes |
2 植物病原细菌的应急反应
植物病原细菌能引起植物的许多重要病害发生,造成农作物减产。植物叶、茎、果实的病斑和坏死是植物病原细菌最常引起的病害症状,病害严重时多个病斑可连成一片,造成植株大片枯死,甚至引起整个器官坏死。双子叶植物病斑因受叶脉限制呈多角形,单子叶植物叶片或茎杆上呈条纹或条板状。许多植物病原细菌病害的病斑呈水渍状,在潮湿环境下,病部表面常产生淡黄色或白色颗粒状、薄膜状的细菌脓,可作为诊断病原细菌病害的特征。目前已发现的植物病原原核生物有30个属,其中研究比较多的革兰氏阴性植物病原细菌有土壤杆菌属(Agrobacterium)、欧氏杆菌属(Erwinia)、假单胞菌属(Pseudomonas)、黄单胞菌属(Xanthomonas)、茄科劳尔氏菌属(Ralstonia)。
2.1 解淀粉欧文氏菌的应急反应解淀粉欧文氏菌能够引起苹果树和梨树的火疫病[30],感染初期,病原菌进入宿主细胞后会面临营养物质匮乏和氧化应激等环境压力,为了度过这些逆境,细菌会合成第二信使——线性核苷酸,如鸟苷四磷酸和鸟苷五磷酸,启动细菌的应急反应,调节与细菌生长和生存相关基因的表达[31]。
Ancona等[30]研究发现,ppGpp缺失突变体(ppGpp0)以及dksA突变体在寄主植物上的致病力和生长繁殖明显降低,表明ppGpp与解淀粉欧文氏菌在寄主植物上的毒性相关。hrp-Ⅲ型分泌系统(Type Ⅲ Secretion System,T3SS)是解淀粉欧文氏菌主要的致病系统,通过抑制宿主的防御系统来调节细菌的早期感染,促进该菌在宿主细胞内繁殖[32]。
Yang等[5]在hrp基因诱导基本培养基(hrp-inducing Minimal Medium,HMM)中分别培养解淀粉欧文氏菌野生型和ppGpp0突变体,将其总RNA提取后进行全基因转录组测序分析,结果发现解淀粉欧文氏菌在营养匮乏的环境中可能利用(p)ppGpp作为信使分子激活致病相关基因的表达,如编码T3SS、胞外多糖、果聚糖、运动等相关基因,同时通过负调控DNA复制、蛋白质翻译、细胞分裂以及核苷酸、氨基酸、脂肪酸和脂质的合成来调节细菌致病和生存之间的平衡。其中,(p)ppGpp通过正调控T3SS相关基因如hrpL、hrpA、hrpN和hrpW的表达,进而调控解淀粉欧文氏菌在寄主植物上的致病力[5](图 2)。
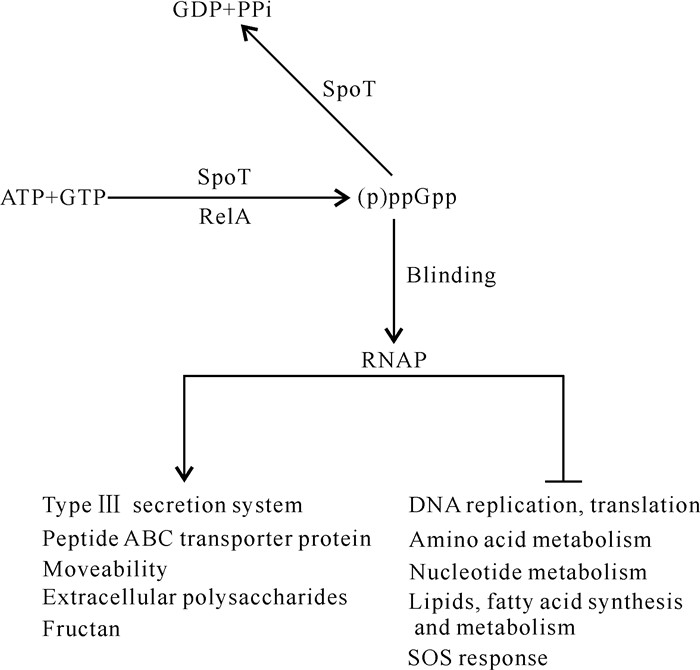
|
| ↓indicates positive regulation, ⊥indicates negative regulation. 图 2 解淀粉欧文氏菌中(p)ppGpp调控模式图 Fig. 2 Diagram of (p)ppGpp regulating pattern in Erwinia amylovora |
2.2 丁香假单胞杆菌的应急反应
丁香假单胞杆菌是许多植物和附生植物重要的病原细菌[6],属于革兰氏阴性植物病原细菌,能适应各种各样的环境,使不同宿主植物如豌豆、卷心菜、黄瓜、番茄、烟草和水稻等致病[33]。根据宿主特异性和病征可将丁香假单胞杆菌分为50多个致病变种,其通过分泌大量致病因子如植物毒素、胞外多糖和T3SS等使宿主致病[34]。丁香假单胞杆菌生活在一些极端环境(如营养物质匮乏、低铁或氧化应激等)时,细胞中的RelA/SpoT会合成ppGpp,从而引发细菌的应急反应。有研究发现,ppGpp在丁香假单胞菌番茄致病变种DC3000(Pseudomonas syringae pv.tomato DC3000,PstDC3000)和丁香假单胞菌致病变种B728a(P.syringae pv.syringae B728a,PssB728a)致病因子的合成与分泌中起着重要的作用[6, 7]。Chatnaparat等[6]在PssB728a中发现,应急反应过程中合成的ppGpp对该菌T3SS相关基因的表达和致病是至关重要的,并且ppGpp还调控该致病菌细胞的大小以及在体外和宿主表面的生存状况。
Liu等[7]在hrp基因诱导基本培养基中分别培养PstDC3000和PssB728a野生型菌株及其各自对应的(p)ppGpp0突变体,通过全基因转录组测序发现,(p)ppGpp正调控多种与致病相关的基因,包括T3SS、Ⅵ型分泌系统(Type Ⅵ Secretion System,T6SS)、细胞运动性、细胞分裂和海藻酸生物合成相关基因;负调控该菌多种生理生化过程,包括DNA复制、核苷酸合成、脂肪酸代谢、核糖体蛋白合成和氨基酸代谢等细胞过程。转录组测序发现,Pst DC3000和PssB728a各自对应的(p)ppGpp0突变体中,有38个T3SS相关基因的表达下调,如hrpL、hrpP-hrpC以及许多效应物相关基因;28个T6SS相关基因的表达下调,如7个ppkA同源基因下调、PssB728a中有15个T6SS相关基因簇HSI-Ⅰ基因表达下调、PstDC3000中有18个T6SS相关基因簇HSI-Ⅱ基因表达下调。(p)ppGpp可通过正调控T3SS和T6SS相关基因的表达,进而影响Pst DC3000和PssB728a在寄主植物上的致病力[7](图 3)。
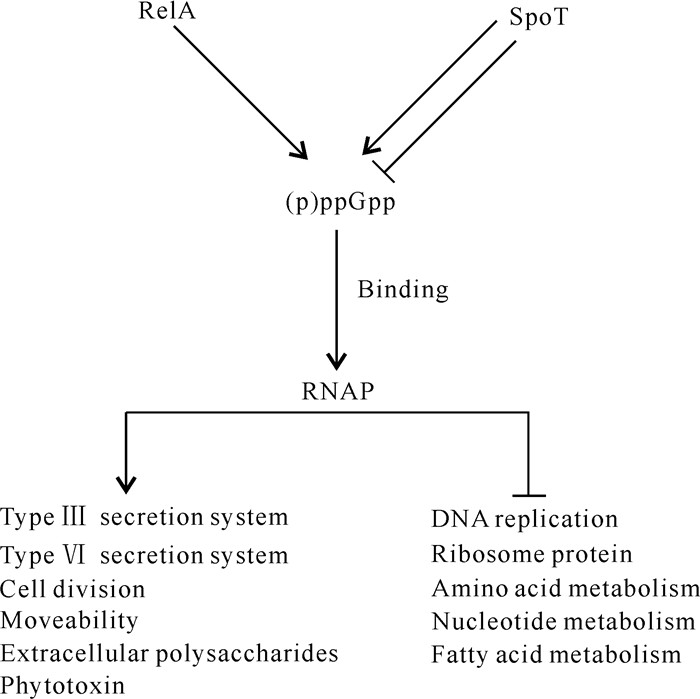
|
| ↓indicates positive regulation, ⊥indicates negative regulation. 图 3 丁香假单胞杆菌中(p)ppGpp的全局效应 Fig. 3 Global effect of (p)ppGpp in Pseudomonas syringae |
2.3 黑腐果胶杆菌的应急反应
黑腐果胶杆菌是革兰氏阴性植物病原菌,通过分泌一些胞外酶降解宿主细胞的细胞壁,引发寄主植物的黑胫病和细菌性软腐病[8, 35]。Toth等[36]研究发现,Pectobacterium atrosepticum strain SCRI1043全基因组中包含许多致病因子编码基因,包括编码蛋白分泌系统的基因、编码植物毒素和细胞壁降解酶的相关基因(如编码果胶裂解酶和外切蛋白酶的基因)。
Wang等[37]研究表明,Pectobacterium atrosepticum strain SCRI1043的(p)ppGpp0突变体ΔrelAΔspoT无法合成植物细胞壁降解酶(Plant Cell Wall Degrading Exoenzymes,PCWDEs)和胞内群体感应信号分子3-氧-己基-L-高丝氨酸内酯(3-Oxo-Hexanoyl-L-Homoserine Lactone,OHHL),而PCWDEs、OHHL与黑腐果胶杆菌的致病力密切相关,因此,(p)ppGpp是调节该致病菌致病的一个重要因子。
2.4 黄单胞菌的应急反应目前在黄单胞菌属中,关于柑橘黄单胞菌柑橘亚种和十字花科黑腐病菌的应急反应报道较多。柑橘黄单胞菌柑橘亚种是革兰氏阴性植物病原细菌,能引起柑橘溃疡病,是柑橘中具有破坏性的细菌病害之一[38]。Zhang等[9]研究发现,转录因子DksA的编码基因dksA突变体ΔdksA和spoT/relA突变体ΔspoTΔrelA在寄主植物上的致病力和生长减弱,DksA和(p)ppGpp抑制了tRNAs和核糖体蛋白编码基因的表达,以及铁离子的吸收和鞭毛的组装,提高了组氨酸代谢、T3SS、Ⅱ型分泌系统(Type Ⅱ Secretion System,T2SS)等相关基因的表达。总之,转录因子DksA和(p)ppGpp在柑橘黄单胞菌柑橘亚种致病、营养吸收和宿主环境适应等过程中起着非常重要的作用。
Xcc属于黄单胞菌属的一个种,其寄主植物为十字花科植物,包括白菜、花椰菜、油菜、芥菜、萝卜等多种十字花科蔬菜。Xcc能导致十字花科植物的黑腐病,被认为是世界范围内十字花科经济作物最重要的病害[39]。T3SS是Xcc重要的致病系统,能将效应物蛋白转运至植物细胞内,使寄主植物致病和非寄主植物产生过敏性反应(Hypersensitive Response,HR)。Xcc8004菌株全基因组中存在relA、spoT同源基因,由于relA、spoT基因双突变体不能合成ppGpp,引起细胞的许多表型发生改变,胞外多糖、胞外酶的产量减少,运动性和致病力丧失[11]。在Xcc中,ppGpp的水平、细胞过程主要由spoT基因调控[11],该基因突变后延迟了Xcc在非寄主植物辣椒ECW-10R上引发的HR反应。研究发现,Xcc中spoT同源基因在寄主植物细胞内外正调控T3SS相关基因的表达,与该菌在寄主植物上的致病力相关[10]。
3 展望应急反应最早在大肠杆菌中提出,是指在氨基酸饥饿时,阻止了rRNA合成的反应[40]。应急反应过程中细菌会迅速合成应急信号分子(p)ppGpp,参与调控细菌的许多表型,如胞外酶的合成、运动性、生物膜的形成、一些毒素的合成、致病力[3, 41-43]等。动物病原细菌与植物病原细菌中均发现,(p)ppGpp可以通过调控Ⅲ型分泌系统和致病因子相关基因的表达,进而影响病原菌的致病力。近年来在一些植物中也发现了参与(p)ppGpp代谢的同源基因,如在拟南芥(Arabidopsis thaliana)中发现了4个RelA/SpoT同源蛋白(Rsh)[44]。
虽然目前关于细菌应急反应以及relA基因、spoT基因功能研究的相关报道较多,但是绝大多数研究多集中在动物病原细菌和一些生防菌中,而在植物病原细菌中应急反应相关的报道还很少。植物病原细菌中(p)ppGpp是一个全局因子,调控病原细菌的致病力以及致病相关的致病因子和致病系统(图 1)。因此,可以推断植物病原细菌应急反应的研究对预防和治理植物细菌病害是非常重要的,(p)ppGpp可作为药物靶点来防治植物细菌病害。尽管目前关于动植物病原细菌应急反应的研究较多,尤其是动物病原细菌应急反应的机制已很明确,但是绝大多数仅停留在理论阶段,将(p)ppGpp作为药物靶点来防治动植物细菌病害的例子仍较少。未来在探究不同植物病原细菌应急反应机制的基础上,还应寻找一些能抑制(p)ppGpp功能的微生物代谢产物,开发出相关生物肥料用于防治植物细菌性病害,减少经济损失。
| [1] |
GRATANI F L, HORVATEK P, GEIGER T, et al. Regulation of the opposing (p)ppGpp synthetase and hydrolase activities in a bifunctional RelA/SpoT homologue from Staphylococcus aureus[J]. PLoS Genetics, 2018, 14(7): e1007514. DOI:10.1371/journal.pgen.1007514 |
| [2] |
WANG B Y, DAI P, DING D, et al. Affinity-based capture and identification of protein effectors of the growth regulator ppGpp[J]. Nature Chemical Biology, 2019, 15(2): 141-150. DOI:10.1038/s41589-018-0183-4 |
| [3] |
SANYAL R, VIMALA A, HARINARAYANAN R. Studies on the regulation of (p)ppGpp metabolism and its perturbation through the over-expression of nudix hydrolases in Escherichia coli[J]. Frontiers in Microbiology, 2020, 11: 562804. DOI:10.3389/fmicb.2020.562804 |
| [4] |
STEINCHEN W, ZEGARRA V, BANGE G. (p)ppGpp: magic modulators of bacterial physiology and metabolism[J]. Frontiers in Microbiology, 2020, 11: 2072. DOI:10.3389/fmicb.2020.02072 |
| [5] |
YANG H W, YU M H, LEE J H, et al. The stringent response regulator (p)ppGpp mediates virulence gene expression and survival in Erwinia amylovora[J]. BMC Genomics, 2020, 21: 261. DOI:10.1186/s12864-020-6699-5 |
| [6] |
CHATNAPARAT T, LI Z, KORBAN S S, et al. The bacterial alarmone (p)ppGpp is required for virulence and controls cell size and survival of Pseudomonas syringae on plants[J]. Environmental Microbiology, 2015, 17(11): 4253-4270. DOI:10.1111/1462-2920.12744 |
| [7] |
LIU J, YU M H, CHATNAPARAT T, et al. Comparative transcriptomic analysis of global gene expression mediated by (p)ppGpp reveals common regulatory networks in Pseudomonas syringae[J]. BMC Genomics, 2020, 21: 296. DOI:10.1186/s12864-020-6701-2 |
| [8] |
BOWDEN S D, EYRES A, CHUNG J C S, et al. Virulence in Pectobacterium atrosepticum is regulated by a coincidence circuit involving quorum sensing and the stress alarmone, (p)ppGpp[J]. Molecular Microbiology, 2013, 90(3): 457-471. DOI:10.1111/mmi.12369 |
| [9] |
ZHANG Y N, TEPER D, XU J, et al. Stringent response regulators (p)ppGpp and DksA positively regulate virulence and host adaptation of Xanthomonas citri[J]. Molecular Plant Pathology, 2019, 20(11): 1550-1565. DOI:10.1111/mpp.12865 |
| [10] |
刘国芳, 苏辉昭, 刘伟, 等. 十字花科黑腐病菌应急反应相关基因spoTXcc对T3SS基因调控作用的分析[J]. 植物病理学报, 2016, 46(1): 27-36. |
| [11] |
BAI K H, YAN H Y, CHEN X, et al. The Role of RelA and SpoT on ppGpp production, stress response, growth regulation, and pathogenicity in Xanthomonas campestris pv.campestris[J]. Microbiology Spectrum, 2021, 9(3): e02057-21. |
| [12] |
DAS B, BHADRA R K. Molecular characterization of Vibrio cholerae ΔrelA ΔspoT double mutants[J]. Archives of Microbiology, 2008, 189: 227-238. DOI:10.1007/s00203-007-0312-z |
| [13] |
SANYAL R, HARINARAYANAN R. Activation of RelA by pppGpp as the basis for its differential toxicity over ppGpp in Escherichia coli[J]. Journal of Biosciences, 2020, 45: 28. DOI:10.1007/s12038-020-9991-2 |
| [14] |
VINELLA D, ALBRECHT C, CASHEL M, et al. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli[J]. Molecular Microbiology, 2005, 56(4): 958-970. DOI:10.1111/j.1365-2958.2005.04601.x |
| [15] |
FITZSIMMONS L F, LIU L, KANT S, et al. SpoT induces intracellular Salmonella virulence programs in the Phagosome[J]. mBio, 2020, 11(1): e03397-19. |
| [16] |
ZHAO Y C, CAI Y Y, CHEN Z H, et al. SpoT-mediated NapA upregulation promotes oxidative stress-induced helicobacter pylori biofilm formation and confers multidrug resistance[J]. Antimicrobial Agents and Chemotherapy, 2021, 65(5): e00152-21. |
| [17] |
HOGG T, MECHOLD U, MALKE H, et al. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response[J]. Cell, 2004, 117(1): 57-68. DOI:10.1016/S0092-8674(04)00260-0 |
| [18] |
NANAMIYA H, KASAI K, NOZAWA A, et al. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis[J]. Molecular Microbiology, 2008, 67(2): 291-304. |
| [19] |
YANG J, ANDERSON B W, TURDIEV A, et al. The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p)ppGpp[J]. Nature Communication, 2020, 11: 5388. DOI:10.1038/s41467-020-19166-1 |
| [20] |
DAS B, PAL R R, BAG S, et al. Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene[J]. Molecular Microbiology, 2009, 72(2): 380-398. DOI:10.1111/j.1365-2958.2009.06653.x |
| [21] |
IHARA Y, MASUDA S. Cytosolic ppGpp accumulation induces retarded plant growth and development[J]. Plant Signal and Behavior, 2016, 11(2): e1132966. DOI:10.1080/15592324.2015.1132966 |
| [22] |
BONIECKA J, PRUSINSKA J, DABROWSKA G B, et al. Within and beyond the stringent response-RSH and (p)ppGpp in plants[J]. Planta, 2017, 246: 817-842. DOI:10.1007/s00425-017-2780-y |
| [23] |
FIEL B. Green magic: regulation of the chloroplast stress response by (p)ppGpp in plants and algae[J]. Journal of Experimental Botany, 2018, 69(11): 2797-2807. DOI:10.1093/jxb/erx485 |
| [24] |
AVILAN L, PUPPO C, VILLAIN A, et al. RSH enzyme diversity for (p)ppGpp metabolism in Phaeodactylum tricornutum and other diatoms[J]. Scientific Reports, 2019, 9(1): 17682. DOI:10.1038/s41598-019-54207-w |
| [25] |
NOMURA Y, NOZAWA A, TOZAWA Y. Biochemical analyses of ppGpp effect on adenylosuccinate synthetases, key enzymes in purine biosynthesis in rice[J]. Bioscience, Biotechnology, and Biochemistry, 2014, 78(6): 1022-1025. DOI:10.1080/09168451.2014.910103 |
| [26] |
SANCHEZ-VAZQUEZ P, DEWEY C N, KITTEN N, et al. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase[J]. Proceedings of the National Academy of Sciences, 2019, 116(17): 8310-8319. DOI:10.1073/pnas.1819682116 |
| [27] |
MAGNUSSON L U, FAREWELL A, NYSTRÖM T. ppGpp: a global regulator in Escherichia coli[J]. Trends in Microbiology, 2005, 13(5): 236-242. DOI:10.1016/j.tim.2005.03.008 |
| [28] |
PACIOS O, BLASCO L, BLERIOT I, et al. (p)ppGpp and its role in bacterial persistence: new challenges[J]. Antimicrobial Agents and Chemotherapy, 2020, 64(10): e01283-20. |
| [29] |
WANG B Y, GRANT R A, LAUB M T. ppGpp coordinates nucleotide and amino-acid synthesis in E.coli during starvation[J]. Molecules and Cells, 2020, 80(1): 29-42. |
| [30] |
ANCONA V, LEE J H, CHATNAPARAT T, et al. The bacterial alarmone (p)ppGpp activates the Type Ⅲ secretion system in Erwinia amylovora[J]. Journal of Bacteriology, 2015, 197(8): 1433-1443. DOI:10.1128/JB.02551-14 |
| [31] |
DALEBROUX Z D, SWANSON M S. ppGpp: magic beyond RNA polymerase[J]. Nature Reviews Microbiology, 2012, 10(3): 203-212. DOI:10.1038/nrmicro2720 |
| [32] |
LEE J H, ZHAO Y F. Integration of multiple stimuli-sensing systems to regulate HrpS and type Ⅲ secretion system in Erwinia amylovora[J]. Molecular Genetics and Genomics, 2018, 293(1): 187-196. DOI:10.1007/s00438-017-1376-3 |
| [33] |
HWANG M S H, MORGAN R L, SARKAR S F, et al. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae[J]. Applied and Environmental Microbiology, 2005, 71(9): 5182-5191. DOI:10.1128/AEM.71.9.5182-5191.2005 |
| [34] |
ISHIGA Y, ICHINOSE Y. Pseudomonas syringae pv.tomato OxyR is required for virulence in tomato and Arabidopsis[J]. Molecular Plant-Microbe Interactions, 2016, 29(2): 119-131. DOI:10.1094/MPMI-09-15-0204-R |
| [35] |
BELL K S, SEBAIHIA M, PRITCHARD L, et al. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp.atroseptica and characterization of virulence factors[J]. Proceedings of the National Academy of Sciences, 2004, 101(30): 11105-11105. DOI:10.1073/pnas.0402424101 |
| [36] |
TOTH I K, PRITCHARD L, BIRCH P R J. Comparative genomics reveals what makes an enterobacterial plant pathogen[J]. Annual Review of Phytopathology, 2006, 44: 305-336. DOI:10.1146/annurev.phyto.44.070505.143444 |
| [37] |
WANG J H, GARDIOL N, BURR T, et al. RelA-dependent (p)ppGpp production controls exoenzyme synthesis in Erwinia carotovora subsp.atroseptica[J]. Journal of Bacteriology, 2007, 189(21): 7643-7652. DOI:10.1128/JB.00920-07 |
| [38] |
VOJNOV A A, DO AMARAL A M, DOW J M, et al. Bacteria causing important diseases of citrus utilise distinct modes of pathogenesis to attack a common host[J]. Applied Microbiology and Biotechnology, 2010, 87: 467-477. DOI:10.1007/s00253-010-2631-2 |
| [39] |
LIU G F, SU H Z, SUN H Y, et al. Competitive control of endoglucanase gene engXCA expression in the plant pathogen Xanthomonas campestris by the global transcriptional regulators HpaR1 and Clp[J]. Molecular Plant Pathology, 2019, 20(1): 51-68. DOI:10.1111/mpp.12739 |
| [40] |
KROL E, BECKER A. ppGpp in Sinorhizobium meliloti: biosynthesis in response to sudden nutritional downshifts and modulation of the transcriptome[J]. Molecular Microbiology, 2011, 81(5): 1233-1254. DOI:10.1111/j.1365-2958.2011.07752.x |
| [41] |
DÍAZ -SALAZAR C, CALERO P, ESPINOSA-PORTERO R, et al. The stringent response promotes biofilm dispersal in Pseudomonas putida[J]. Scientific Report, 2017, 7: 18055. DOI:10.1038/s41598-017-18518-0 |
| [42] |
PRUSA J, ZHU D X, STALLINGS C L. The stringent response and Mycobacterium tuberculosis pathogenesis[J]. Pathogens and Disease, 2018, 76(5): fty054. DOI:10.1093/femspd/fty054 |
| [43] |
KIM K, ISLAM M, JUNG H W, et al. ppGpp signaling plays a critical role in virulence of Acinetobacter baumannii[J]. Virulence, 2021, 12(1): 2122-2132. DOI:10.1080/21505594.2021.1961660 |
| [44] |
MIZUSAWA K, MASUDA S, OHTA H. Expression profiling of four RelA/SpoT-like proteins, homologues of bacterial stringent factors, in Arabidopsis thaliana[J]. Planta, 2008, 228: 553-562. DOI:10.1007/s00425-008-0758-5 |



