棘皮动物是海洋底栖无脊椎动物中的重要类群,蛇尾纲(Ophiuroidea)在棘皮动物门(Echinodermata)中种类最多、资源丰富。蛇尾纲动物是中国海域最丰富的棘皮动物种群,共89属221种[1]。据报道,蛇尾纲动物含有多糖、甾族类化合物、皂苷、不饱和脂肪酸、倍半萜、神经节苷脂等成分,这些成分具有一定的抗肿瘤、抗病毒、抗菌、抗血管形成、抗氧化等活性[2-7],但蛇尾纲动物隐板裸刺蛇尾(Gymnolophus obscura)的化学成分研究还未见报道。
有研究表明,从无脊椎动物中分离得到的许多化合物是由其共附生微生物产生的[8-10]。据统计,海参来源可培养真菌属于子囊菌门(Ascomycota)24科29属,并能够产生聚酮类、生物碱和萜类等化合物,这些化合物具有细胞毒、抗菌、抗血管生成和酶抑制等多种生物活性[11]。海星的共附生真菌研究报道较多,其次级代谢产物结构具有化学多样性,并具有抗菌和抗肿瘤等活性[12-17]。海百合可培养共附生真菌包括小光壳属(Leptosphaerulina)、链格孢属(Alternaria)、曲霉属(Aspergillus),其次级代谢产物也有众多的研究报道[18-20]。海胆共附生真菌的次级代谢产物具有抗菌和细胞毒活性[21, 22]。但是国内外有关蛇尾纲的共附生真菌及活性物质方面的研究未见报道。为进一步阐明蛇尾纲动物共附生真菌的多样性,为其天然活性代谢产物研究提供微生物资源,本研究从西沙群岛采集隐板裸刺蛇尾,分离其可培养共附生真菌,并研究其共附生真菌代谢产物的抑菌活性,以丰富人们对隐板裸刺蛇尾共附生微生物的认知,为其微生物资源开发利用提供菌种基础,同时为海洋微生物的药用开发利用提供一定的科学依据。
1 材料与方法 1.1 材料 1.1.1 供试动物隐板裸刺蛇尾于2020年7月采自西沙群岛,采样后装入保鲜袋,于冰盒中保存,24 h内送至实验室保存在4 ℃冰箱。参考《中国动物志: 无脊椎动物第四十卷棘皮动物门蛇尾纲》[23]的特征描述鉴定其为隐板裸刺蛇尾。
1.1.2 培养基微生物分离所用培养基包括察氏培养基、MB培养基(麦芽提取粉15.0 g,海盐15.0 g,琼脂20.0 g,氯霉素和氨苄青霉素各100 mg,蒸馏水1 L)和孟加拉红琼脂培养基。抑菌活性测试所用培养基为Luria-Bertani (LB)固体培养基和营养肉汤(NB)固体培养基,不加琼脂则为液体培养基。大米培养基(大米100 g,海盐2 g,蒸馏水110 mL)用于共附生真菌的发酵。
1.1.3 指示菌铜绿假单胞菌(Pseudomona aeruginosa ATCC10145)、耐甲氧西林金黄色葡萄球菌(Methicillin-resistant Staphylococcus aureus ATCC43300,MRSA)、表皮葡萄球菌(Staphylococcus epidermidis ATCC12228)、Actinomyces viscosus ATCC15987、肺炎克雷伯菌(Klebsiella pneumoniae ATCC13883)、鲍曼不动杆菌(Acinetobacter baumannii ATCC19606)由广西中医药大学海洋药物研究院保藏。
1.1.4 主要试剂和仪器氯仿、乙酸乙酯、二甲基亚砜(DMSO)试剂等均为分析纯,广东光华科技股份有限公司生产;引物ITS1/ITS4,生工生物工程(上海)股份有限公司合成;DNA提取试剂盒(DP305),天根生化科技(北京)有限公司生产;PCR试剂盒(2×EasyTaq PCR Super Mix),北京全式金生物技术股份有限公司生产;PCR仪(S1000型),美国Bio-Rad公司生产;电泳仪(DYCP-31E型),北京六一生物科技有限公司生产;凝胶成像分析仪(INFINITY 3026型),VILBER LOURMAT公司生产;培养箱(SHP-150型),上海精宏实验设备有限公司生产;旋转蒸发仪(N-1300D-WB型),日本东京理化器械株式会社生产。
1.2 方法 1.2.1 共附生真菌的分离用无菌水清洗隐板裸刺蛇尾表面3遍,加入5 mL无菌海盐水研磨,研磨后的液体视为样品原液,保存于离心管备用。样品原液稀释至10-2、10-3和10-4倍后,均匀涂布到相应的3种分离培养基平板上,于25 ℃培养箱培养,自第5天开始每日观察菌落生长情况,并将其转接到另一新的平板上进行三区划线纯化。记录菌落数及菌落的形态特征。
1.2.2 菌株鉴定取新鲜菌体,液氮研磨,按照DNA提取试剂盒的说明提取真菌基因组。采用真菌通用引物ITS1(5′-TCCGTAGGTGAACCTGCGG-3′)和ITS4 (5′-TCCTCCGCTTATTGATATGC-3′)进行PCR扩增。扩增样品经琼脂糖凝胶电泳验证后,委托生工生物工程(上海)股份有限公司进行测序分析。测序结果提交至GenBank数据库,应用Blast进行序列相似性比对,获得相似度高的种属信息。使用MEGE-X软件的UPGMA法构建系统发育树,置信值检测设为Bootstrap 1 000次。
1.2.3 共附生真菌代谢产物的制备将分离得到的菌株接种到大米培养基,25 ℃静置培养30 d。使用乙酸乙酯作为溶剂,超声提取代谢产物3次,合并提取液并减压浓缩,回收溶剂,得到共附生真菌代谢产物。
1.2.4 共附生真菌代谢产物的抑菌活性测试利用DMSO溶解代谢产物,样品浓度为50 mg·mL-1,备用。指示菌耐甲氧西林金黄色葡萄球菌、铜绿假单胞菌、表皮葡萄球菌、肺炎克雷伯菌培养于LB培养基,Actinomyces viscosus、鲍曼不动杆菌培养于NB培养基。采用96孔板法测定发酵产物对指示菌的抑制活性。96孔板的第1列加入190 μL灭菌液体培养基,第2-11列加入100 μL灭菌液体培养基;然后再往第1列中加入10 μL样品溶液,混合均匀,取100 μL加入到第2列,采用倍比稀释法稀释至第9列。第1-9列的每孔加入100 μL菌液,混合均匀,使得对应列的样品终浓度分别为1.250 00、0.625 00、0.312 50、0.156 25、0.078 13、0.039 06、0.019 53、0.009 77、0.004 88 mg·mL-1。第10列加入100 μL灭菌液体培养基代替菌液作为阴性对照,第11列加入100 μL菌液但不加测试样品作为空白对照。阳性药物氨苄青霉素钠和环丙沙星终浓度均为50.000、25.000、12.500、6.250、3.125、1.563、0.781、0.391、0.195 μg·mL-1。置37 ℃恒温培养箱培养24 h,观察实验现象。如孔内液体呈浑浊状态,说明相应浓度的样品无抗菌活性;若孔内液体澄清,说明小孔内细菌生长被抑制。对应澄清孔的最小样品浓度为发酵产物抑制致病菌的最低抑制浓度(MIC)。
2 结果与分析 2.1 共附生真菌的分离从隐板裸刺蛇尾共分离出25株可培养共附生真菌。根据菌落形态特征,这25株真菌可初步分为11种,其菌落培养特征如图 1所示。从孟加拉红琼脂培养基中可分离出菌株GXIMD02016、GXIMD02017、GXIMD02018、GXIMD02019、GXIMD02023,MB培养基中可分离出菌株GXIMD02018、GXIMD02020、GXIMD02021、GXIMD02022、GXIMD02023,察氏培养基中可分离出菌株GXIMD02017、GXIMD02023、GXIMD02024、GXIMD02025、GXIMD02026。将菌株纯化至平板上供后续实验使用。
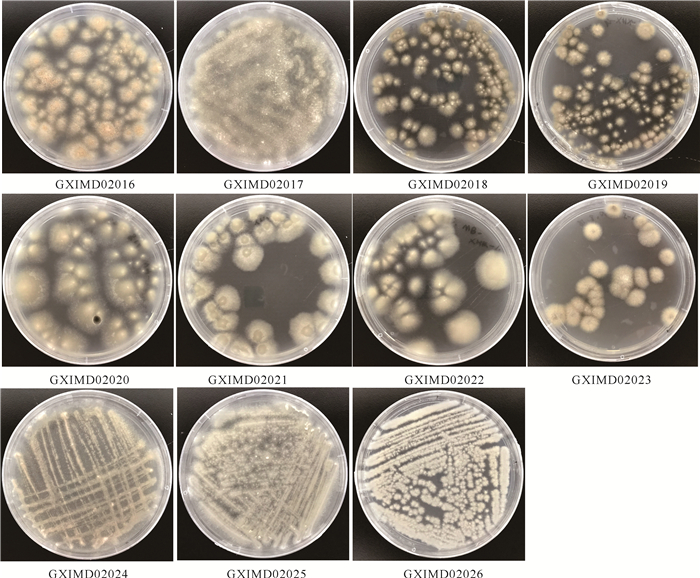
|
| 图 1 隐板裸刺蛇尾共附真菌在MB培养基上的菌落形态 Fig. 1 Colony morphology cultured on MB medium of symbiotic and epiphytic fungi from Gymnolophus obscura |
2.2 菌株鉴定
通过Blast比对ITS rDNA基因序列的结果如表 1所示。GXIMD02021和GXIMD02022株菌与已确定分类地位和未确定分类地位菌株的相似度均不到97%,为潜在新菌, 占已鉴定菌株数量的18.2%;GXIMD02016、GXIMD02020与已确定分类地位菌株的序列同源性最高相似度小于97%,但与未确定分类地位的格孢腔菌目(Pleosporales)菌株序列同源性最高相似度大于98%;其他菌株与已报道菌株的相似度均大于99%,包括3株枝顶孢属(Acremonium)菌株、2株Trematosphaeria属菌株、1株拟盾壳霉属(Paraconiothyrium)菌株、1株丛赤壳属(Nectria)菌株。
| 菌株编号 Strain code |
相似菌株 Similar strain |
相似菌株登录号 Similar strain registration number |
相似度/% Similarity/% |
| GXIMD02016 | Pleosporales sp. | MH141290.1 | 98.82 |
| Tremateia chiangraiensis | NR168867.1 | 93.76 | |
| GXIMD02017 | Acremonium alternatum | MK952527.1 | 99.81 |
| GXIMD02018 | Trematosphaeria grisea | KX757030.1 | 99.80 |
| GXIMD02019 | Paraconiothyrium cyclothyrioides | MF135511.1 | 100.00 |
| GXIMD02020 | Pleosporales sp. | MK732149.1 | 100.00 |
| Bimuria novae-zelandiae | OM337538.1 | 92.61 | |
| GXIMD02021 | Leptosphaerulina sp. | KY827352.1 | 94.98 |
| Letendraea helminthicola | MH483996.1 | 91.77 | |
| GXIMD02022 | Leptosphaerulina sp. | KY827352.1 | 96.69 |
| Pseudopithomyces angolensis | NR161121.1 | 93.19 | |
| GXIMD02023 | Trematosphaeria grisea | KT220662.1 | 99.60 |
| GXIMD02024 | Acremonium sclerotigenum | MT226598.1 | 99.80 |
| GXIMD02025 | Nectria dematiosa | MH748176.1 | 99.03 |
| GXIMD02026 | Acremonium alternatum | MT529342.1 | 99.80 |
应用MEGE-X软件中的Clustal W模块进行DNA排序,用UPGMA法构建系统发育树(图 2)。从图 2可以看出,11株真菌与序列同源性最高菌株构成了2个大聚类,分别属于格孢腔菌目(Pleosporales)和肉座菌目(Hypocreales)。GXIMD02016与Pleosporales sp.(MH141290.1)、Tremateia chiangraiensis(NR168867.1)形成了一个100%支持率的聚类,且与未确定分类地位的格孢腔菌目真菌Pleosporales sp.(MH141290.1)形成了末端聚类,说明GXIMD02016与格孢腔菌目(Pleosporales)隔孢假壳科(Didymosphaeriaceae)的真菌亲缘关系密切,但尚不能具体到属和种。GXIMD02021与未确定分类地位真菌Leptosphaerulina sp.(KY827352.1)、Pleosporales sp.(MK732149.1)构成了77%支持率的聚类。GXIMD02020、GXIMD02022分别与Pleosporales sp.(MK732149.1)和Leptosphaerulina sp.(KY827352.1)形成末端聚类。虽然GXIMD02020、GXIMD02021、GXIMD02022 ITS rDNA基因序列分别与Bimuria novae-zelandiae(OM337538.1)、Letendraea helminthicola(MH483996.1)、Pseudopithomyces angolensis(NR161121.1)相似度较高,但是没有形成末端聚类。目前,GXIMD02020、GXIMD02021、GXIMD02022尚不能解析到具体的种,但是从系统发育树(图 2)可知,它们与隔孢假壳科(Didymosphaeriaceae)的真菌亲缘关系密切。其他菌株与ITS rDNA基因序列高度相似的菌株均构成了末端聚类,且支持率为54%-100%。

|
| 图 2 运用UPGMA法基于ITS rDNA序列构建的系统发育树 Fig. 2 Phylogenetic tree constructed by UPGMA method based on the ITS rDNA sequence |
2.3 共附生真菌的抑菌活性
隐板裸刺蛇尾共附生真菌代谢产物抗菌活性如表 2所示。GXIMD02016、GXIMD02017、GXIMD02018、GXIMD02021、GXIMD02024、GXIMD02025、GXIMD02026菌株代谢产物有一定的抗表皮葡萄球菌活性,其MIC值为0.312 5-1.250 0 mg·mL-1。GXIMD02018、GXIMD02020、GXIMD02021、GXIMD02022代谢产物抑制MRSA的MIC值为0.625 0-1.250 0 mg·mL-1。GXIMD02022和GXIMD02024菌株代谢产物显著抑制铜绿假单胞菌,MIC值为0.039 0 mg·mL-1;GXIMD02021菌株代谢产物抑制铜绿假单胞菌的MIC值为1.250 0 mg·mL-1。除GXIMD02017和GXIMD02021外,其他菌株代谢产物均具有一定的抑制Actinomyces viscosus活性,其中GXIMD02019和GXIMD02025菌株代谢产物具有显著的抑制Actinomyces viscosus的活性,MIC值为0.156 mg·mL-1。所有测试样品未显示有抑制肺炎克雷伯菌和鲍曼不动杆菌活性。
| Unit: mg·mL-1 | |||||||||||||||||||||||||||||
| 菌株Strain | MIC value | ||||||||||||||||||||||||||||
| Staphylococcus epidermidis | MRSA | Pseudomona aeruginosa | Actinomyces viscosus | Klebsiella pneumoniae | Acinetobacter baumannii | ||||||||||||||||||||||||
| GXIMD02016 | 0.625 00 | - | - | 1.250 00 | - | - | |||||||||||||||||||||||
| GXIMD02017 | 0.312 50 | - | - | - | - | - | |||||||||||||||||||||||
| GXIMD02018 | 1.250 00 | 1.250 00 | - | 0.625 00 | - | - | |||||||||||||||||||||||
| GXIMD02019 | - | - | - | 0.156 25 | - | - | |||||||||||||||||||||||
| GXIMD02020 | - | 1.250 00 | - | 0.625 00 | - | - | |||||||||||||||||||||||
| GXIMD02021 | 0.625 00 | 1.250 00 | 1.250 00 | - | - | - | |||||||||||||||||||||||
| GXIMD02022 | - | 0.625 00 | 0.039 06 | 0.312 50 | - | - | |||||||||||||||||||||||
| GXIMD02023 | - | - | - | 0.625 00 | - | - | |||||||||||||||||||||||
| GXIMD02024 | 0.625 00 | - | 0.039 06 | 1.250 00 | - | - | |||||||||||||||||||||||
| GXIMD02025 | 0.312 50 | - | - | 0.156 25 | - | - | |||||||||||||||||||||||
| GXIMD02026 | 1.250 00 | - | - | 1.250 00 | - | - | |||||||||||||||||||||||
| Ampicillin sodium | 3.125 | 3.125 | - | 6.250 | 1.563 | 1.563 | |||||||||||||||||||||||
| Ciprofloxacin | 6.250 | 0.781 | 3.125 | 3.125 | 0.781 | 3.125 | |||||||||||||||||||||||
3 讨论
本研究开展蛇尾纲动物共附生真菌研究,从隐板裸刺蛇尾分离25株共附生真菌,按其形态特征归属为11种。GXIMD02016、GXIMD02019仅从孟加拉红琼脂培养基中分离得到;GXIMD02020、GXIMD02021、GXIMD02022仅从MB培养基中分离得到;GXIMD02024、GXIMD02025、GXIMD02026仅从察氏培养基中分离得到,说明不同的培养基对海洋真菌有选择性且有助于丰富真菌的多样性,与刘思远等[24]的研究结论相似。
本研究分离得到的9株真菌与已报道真菌的基因序列相似度大于98%,且形成末端聚类。此外,GXIMD02021和GXIMD02022菌株在Blast分析时与确定分类地位菌株的相似度均小于97%,且没有形成末端聚类。通常相似度94.9%-98.0%或平均相似度96.4%被认为是区分新种的界限[25],GXIMD02021和GXIMD02022与其他菌株相似度小于97%,因而无法确定其种,为潜在新菌种,在后续研究中可根据形态特征以及基于其他基因序列构建的系统发育树进一步确定其归类。GXIMD02016和GXIMD02020菌株与未确定分类地位的格孢腔菌目菌株基因序列相似度大于98%且在系统发育树中与其形成末端聚类,但与已确定分类地位菌株的最高相似度小于94%,其种属归类需要进一步确认。
海洋微生物来源的天然产物是海洋新药开发的研究热点,主要来自海洋真菌和海洋细菌。然而,最新统计分析表明70%海洋真菌天然产物与已知化合物相似,新颖结构类型化合物较少,研究海洋特定且未研究的微生物有利于发现结构新颖的化合物[26]。本研究已获得两株潜在新菌GXIMD02021和GXIMD02022,今后可对其化学成分开展研究以期获得结构新颖的海洋天然产物。本研究分离得到了枝顶孢属(Acremonium)、Trematosphaeria属、拟盾壳霉属(Paraconiothyrium)、丛赤壳属(Nectria)的菌株,这些属菌的代谢产物具有抗菌、抗肿瘤、免疫抑制、抗氧化、抗炎、抗疟疾、细胞毒性、植物毒性、抗病毒、神经保护、杀虫和酶抑制等多种生物活性[27-32],有着广泛的药用前景。因此,本研究分离得到的菌株可以为发掘生物活性物质提供微生物资源。
表皮葡萄球菌、MRSA、铜绿假单胞菌是临床致病菌,本研究结果表明隐板裸刺蛇尾共附生真菌的代谢产物对表皮葡萄球菌、MRSA、铜绿假单胞菌有一定的抑制作用。因此,隐板裸刺蛇尾共附生真菌是发掘抗菌活性物质的资源,具体的抗菌成分有待于进一步研究。Actinomyces viscosus是菌斑的主要成员,与龋病以及牙周病的发生有关,隐板裸刺蛇尾共附生真菌的代谢产物对其有一定的抑制作用,具有开发成口腔护理产品的潜在价值。
4 结论隐板裸刺蛇尾共附生真菌属于格孢腔菌目真菌和肉座菌目真菌,包括枝顶孢属、Trematosphaeria属、拟盾壳霉属、格孢腔菌属和丛赤壳属菌株。潜在新菌的数量占已分离菌株的18.2%,比例较高。隐板裸刺蛇尾共附生真菌的代谢产物有一定的抗菌活性。本研究进一步丰富了棘皮动物共附生真菌的多样性,完善了棘皮动物门共附生真菌样品库,为隐板裸刺蛇尾共附生真菌次级代谢产物及其生物活性研究提供了菌种来源,有利于海洋药用微生物资源的开发。
| [1] |
史博洋, 鲁亚莉, 潘雅君, 等. 蛇尾纲生物学研究进展[J]. 琼州学院学报, 2016, 23(2): 92-98. |
| [2] |
ANDERSSON L, BOHLIN L, IORIZZI M, et al. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars[J]. Toxicon, 1989, 27(2): 179-188. DOI:10.1016/0041-0101(89)90131-1 |
| [3] |
MANSOUR M P, HOLDSWORTH D G, FORBES S E, et al. High contents of 24∶6(n-3) and 20∶1(n-13) fatty acids in the brittle star Amphiura elandiformis from Tasmanian coastal sediments[J]. Biochemical Systematics and Ecology, 2005, 33(7): 659-674. DOI:10.1016/j.bse.2004.12.011 |
| [4] |
NUZZO G, GOMES B A, AMODEO P, et al. Isolation of chamigrene sesquiterpenes and absolute configuration of isoobtusadiene from the brittle star Ophionereis reticulata[J]. Journal of Natural Products, 2017, 80(11): 3049-3053. DOI:10.1021/acs.jnatprod.7b00510 |
| [5] |
甄静荣, 丛建森, 刘艳萍, 等. 金氏真蛇尾皂苷的抗氧化和抑菌活性研究[J]. 食品科技, 2015, 40(6): 285-288. |
| [6] |
AMININ D L, AGAFONOVA I G, FEDOROV S N. Biological activity of disulfated polyhydroxysteroids from the pacific brittle star Ophiopholis aculeata[J]. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 1995, 112(2): 201-204. DOI:10.1016/0742-8413(95)02012-8 |
| [7] |
AMINI E, NABIUNI M, BAHARARA J, et al. In-vitro pro apoptotic effect of crude saponin from Ophiocoma erinaceus against cervical cancer[J]. Iranian Journal of Pharmaceutical Research, 2017, 16(1): 266-276. |
| [8] |
SIMMONS T L, COATES R C, CLARK B R, et al. Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(12): 4587-4594. |
| [9] |
KÖNIG G M, KEHRAUS S, SEIBERT S F, et al. Natural products from marine organisms and their associated microbes[J]. Chembiochem, 2006, 7(2): 229-238. DOI:10.1002/cbic.200500087 |
| [10] |
HENTSCHEL U, PIEL J, DEGNAN S M, et al. Genomic insights into the marine sponge microbiome[J]. Nature Reviews Microbiology, 2012, 10(9): 641-654. DOI:10.1038/nrmicro2839 |
| [11] |
CHEN L, WANG X Y, LIU R Z, et al. Culturable microorganisms associated with sea cucumbers and microbial natural products[J]. Marine Drugs, 2021, 19(8): 461. DOI:10.3390/md19080461 |
| [12] |
WEI X, FENG C, LI X H, et al. Enantiomeric polyketides from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2[J]. Chemistry & Biodiversity, 2019, 16(6): e1900052. |
| [13] |
YAN D F, LAN W J, WANG K T, et al. Two chlorinated benzofuran derivatives from the marine fungus Pseudallescheria boydii[J]. Natural Product Communications, 2015, 10(4): 621-622. |
| [14] |
LIANG W L, LE X, LI H J, et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri[J]. Marine Drugs, 2014, 12(11): 5657-5676. DOI:10.3390/md12115657 |
| [15] |
LAN W J, LIU W, LIANG W L, et al. Pseudaboydins A and B: novel isobenzofuranone derivatives from marine fungus Pseudallescheria boydii associated with starfish Acanthaster planci[J]. Marine Drugs, 2014, 12(7): 4188-4199. DOI:10.3390/md12074188 |
| [16] |
NIU X, DAHSE H M, MENZEL K D, et al. Butyrolactone I derivatives from Aspergillus terreus carrying an unusual sulfate moiety[J]. Journal of Natural Products, 2008, 71(4): 689-692. DOI:10.1021/np070341r |
| [17] |
刘威, 梁婉玲, 谢中梁, 等. 南海长棘海星共附生真菌的分离鉴定及多样性分析[J]. 中山大学学报(自然科学版), 2015, 54(1): 84-88, 95. |
| [18] |
ZHANG P P, LI J, LANG J J, et al. Two new sesquiterpenes derivatives from marine fungus Leptosphaerulina Chartarum sp. 3608[J]. Natural Product Research, 2018, 32(19): 2297-2303. DOI:10.1080/14786419.2017.1408102 |
| [19] |
ZHANG L H, WANG H W, XU J Y, et al. A new secondary metabolites of the crinoid (Comanthina schlegeli) associated fungus Alternaria brassicae 93[J]. Natural Product Research, 2016, 30(20): 2305-2310. DOI:10.1080/14786419.2016.1166498 |
| [20] |
LI Y F, WU X B, NIAZ S I, et al. Effect of culture conditions on metabolites produced by the crinoid-derived fungus Aspergillus ruber 1017[J]. Natural Product Research, 2017, 31(11): 1299-1304. DOI:10.1080/14786419.2016.1244200 |
| [21] |
WANG R, LIU T M, SHEN M H, et al. Spiculisporic acids B-D, three new γ-butenolide derivatives from a sea urchin-derived fungus Aspergillus sp. HDf2[J]. Molecules, 2012, 17(11): 13175-13182. DOI:10.3390/molecules171113175 |
| [22] |
YAMADA T, TANAKA A, NEHIRA T, et al. Altercrasins A-E, decalin derivatives, from a sea-urchin-derived Alternaria sp. : isolation and structural analysis including stereochemistry[J]. Marine Drugs, 2019, 17(4): 218. DOI:10.3390/md17040218 |
| [23] |
廖玉麟. 中国动物志: 无脊椎动物第四十卷棘皮动物门蛇尾纲[M]. 北京: 科学出版社, 2004.
|
| [24] |
刘思远, 王巧贞, 吴鸿仙, 等. 普通培养基与优化培养基对防城港海域真菌分离效果的影响[J]. 广西科学, 2021, 28(1): 65-73. |
| [25] |
YARZA P, RICHTER M, PEPLIES J, et al. The all-species living tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains[J]. Systematic and Applied Microbiology, 2008, 31(4): 241-250. DOI:10.1016/j.syapm.2008.07.001 |
| [26] |
VOSER T M, CAMPBELL M D, CARROLL A R. How different are marine microbial natural products compared to their terrestrial counterparts?[J]. Natural Product Reports, 2022, 39(1): 7-19. DOI:10.1039/D1NP00051A |
| [27] |
TIAN J, LAI D W, ZHOU L G. Secondary metabolites from Acremonium Fungi: diverse structures and bioactivities[J]. Mini Reviews in Medicinal Chemistry, 2017, 17(7): 603-632. DOI:10.2174/1389557516666160914194134 |
| [28] |
WANG J F, SHAO S C, LIU C S, et al. The genus Paraconiothyrium: species concepts, biological functions, and secondary metabolites[J]. Critical Reviews in Microbiology, 2021, 47(6): 781-810. DOI:10.1080/1040841X.2021.1933898 |
| [29] |
YU H B, JIAO H, ZHU Y P, et al. Bioactive metabolites from the Arctic fungus Nectria sp. B-13[J]. Journal of Asian Natural Products Research, 2019, 21(10): 961-969. DOI:10.1080/10286020.2018.1482880 |
| [30] |
CUI H, LIU Y Y, NIE Y, et al. Polyketides from the mangrove-derived endophytic fungus Nectria sp. HN001 and their α-glucosidase inhibitory activity[J]. Marine Drugs, 2016, 14(5): 86. DOI:10.3390/md14050086 |
| [31] |
ZHANG P P, JIA C X, LANG J J, et al. Mono- and dimeric naphthalenones from the marine-derived fungus Leptosphaerulina chartarum 3608[J]. Marine Drugs, 2018, 16(5): 173. DOI:10.3390/md16050173 |
| [32] |
SONG B, LI L Y, SHANG H, et al. Trematosphones A and B, two unique dmeric structures from the desert plant endophytic fungus Trematosphaeria terricola[J]. Organic Letters, 2019, 21(7): 2139-2142. DOI:10.1021/acs.orglett.9b00454 |



