2. 广西壮族自治区中国科学院广西植物研究所,广西喀斯特植物保育与恢复生态学重点实验室,广西桂林 541006
2. Guangxi Key Laboratory of Plant Conservation and Restoration Ecology in Karst Terrain, Guangxi Institute of Botany, Guangxi Zhuang Autonomous Region and Chinese Academy of Sciences, Guilin, Guangxi, 541006, China
随着有机发光显示技术的迅速发展,各种有机发光材料(Organic Light-Emitting Materials,OLEMs)被广泛应用于光电显示、化学传感、生物成像[1-3]等领域,图 1展示了9种典型的OLEMs的分子结构。部分OLEMs具有与多环芳烃类似的稠环结构,且含有氟、氯、溴等卤素原子[4],可能具有致癌、致畸和急性毒性等毒理学性质。近期也有研究在室内灰尘、沉积物和水生生物中检测到OLEMs[5-8], 说明OLEMs可能在生产、运输、储存、使用或处置过程中进入环境。Zheng等[9]通过定量分析,在母乳样品中检测到部分OLEMs。前人研究已充分说明OLEMs已进入人体且极大可能对人体健康造成潜在危害。根据监管规定,化学品供应商有义务对所生产销售化学品的理化及毒理性质进行必要检测并对其危害特性进行分类和标识,从而确保化学品的安全使用、运输和处置[10, 11]。欧洲化学品管理局(European Chemicals Agency,ECHA)建立维护的化学品分类和标签数据库(Classification and Labelling Inventory,C & L清单)保存有供应商提交的化学品危害特性分类信息,这为综合探讨OLEMs对人体健康危害性提供了一手资料。目前对OLEMs的毒理学研究存在不足,且现有定量构效关系(Quantitative Structure-Property Relationship,QSPR)模型不能准确预测OLEMs的毒理性质。基于此,本研究提出OLEMs对人体健康危害的预测模型,为优先控制化学品的筛选提供依据。
|
| (a) 3, 5-Diphenyl-4-(1-naphthyl)-1H-1, 2, 4-triazole; (b) 2, 3, 5, 6-Tetrafluoro-7, 7′, 8, 8′-tetracyanoquinodimethane; (c) 1, 2-Dichloro-4, 5-dicyanobenzoquinone; (d) 1, 4-Bis(diphenylamino)benzene; (e) Tris(4-bromophenyl)amine; (f) 2, 4, 6-Tris(4-pyridinyl)-1, 3, 5-triazine; (g) 5, 6, 11, 12-Tetraphenylnaphthacene; (h) 29H, 31H-Phthalocyanine; (i) 1, 3, 6, 8-Tetraphenylpyrene. 图 1 典型有机发光材料分子结构示意 Fig. 1 Molecular structures of typical organic light-emitting materials |
1 材料与方法 1.1 数据库的建立
通过Free Patents Online (https://www.freepatentsonline.com/)检索用于制作有机发光二极管的OLEMs,建立了包含1 988种OLEMs的化学品清单,其中94种的分类与标签信息已被C & L清单收录。通过ChemDraw v20.0.0.41和PubChem(https://pubchem.ncbi.nlm.nih.gov/)获取分子结构的简化分子线性输入规范(SMILES)表达式,采用Open Babel工具箱(v3.1.1,http://openbabel.org/)转换分子格式,利用PaDEL-Descriptor (v2.21,http://padel.nus.edu.sg/)提取出1 875种分子描述符,通过SPSS v26.0对分子描述符进行相关性分析和初步筛选,筛选过程如下:1)删除对于大部分化合物数值都为0的分子描述符;2)对服从正态分布的分子描述符进行单因素方差分析,对非正态分布的分子描述符进行非参数检验;3)对通过单因素方差分析(P < 0.1)和非参数检验(P < 0.1)的分子描述符进行二元Logistic回归分析,筛选出通过回归分析(P < 0.05)的分子描述符。采用卡方检验评估有机发光材料的健康危害特性之间的相关性[12],同时根据OLEMs暴露途径和造成健康危害的程度不同,将94种OLEMs的健康危害分为接触性危害和吸入性危害。最终用于构建接触危害性模型的分子描述符为38种,用于构建吸入危害性模型的分子描述符为67种。
1.2 定量构效关系模型的构建将C & L清单收录的94种OLEMs随机分为训练集(70%)和测试集(30%)。训练集用于模型的学习,模型系数通过GMDH Shell v3.8.2拟合,通过10折交叉验证提高模型泛化能力并减少误差。测试集用于评估模型性能,评价指标包括准确率(ACC)、敏感性(SEN)、特异性(SPE)、阳性预测值(PPV)、阴性预测值(NPV)[13]。
| $ {\rm{ACC}} = (TP + TN)/(TP + TN + FP + FN) $ | (1) |
| $ {\rm{SEN}} = TP/(TP + FN) $ | (2) |
| $ {\rm{SPE}} = TN/(TN + FP) $ | (3) |
| $ {\rm{PPV}} = TP/(TP + FP) $ | (4) |
| $ {\rm{NPV}} = TN/(TN + FN) $ | (5) |
式中,TP为真阳性预测数;TN为真阴性预测数;FP为假阳性预测数;FN为假阴性预测数。
1.3 定量构效关系模型与现有模型的比较分别应用STopTox(https://stoptox.mml.unc.edu/)、VEGA v1.2.3、QSAR Toolbox v4.6和RespiraTox(https://respiratox.item.fraunhofer.de/)等模型对C & L清单收录的94种OLEMs的接触及吸入危害性进行预测。其中,通过STopTox模型预测对皮肤的腐蚀性、刺激性及致敏性,对眼晴的刺激性和急性吸入毒性;通过VEGA模型预测对皮肤的刺激性、致敏性和对眼晴的刺激性;通过QSAR Toolbox模型预测对皮肤及呼吸道的致敏性;通过RespiraTox模型预测对呼吸道的致敏性。通过对比新构建的QSPR模型与现有模型的预测准确率、敏感性、特异性、阳性预测值和阴性预测值,对QSPR模型的预测能力进行评价。
1.4 定量构效关系模型应用域的定义模型的应用域(Applicability Domain,AD)是包含了训练集各种结构特征的空间,模型能够可靠地预测应用域内的化合物[14]。一般认为,QSPR模型对相似分子的预测是可靠的[15]。本研究基于Tanimoto系数和二进制指纹来测量分子间的相似程度,设定相似阈值为90%,通过PubChem数据库检索出与OLEMs具有相似结构且被C & L清单收录的200个类OLEMs分子作为外部数据集。分别采用基于欧氏距离[15]、分子描述符标准化[16]及核密度估计[17]的方法定义QSPR模型的应用域。其中,定义D1为新化合物与训练集中所有化合物之间的平均欧氏距离,D2表示训练集中化合物之间的平均欧氏距离,若D1≤D2,则认为新化合物处于应用域内;定义Ski为新化合物k的分子描述符i基于训练集中化合物标准化后的值,若Ski的最大值≤3,则认为化合物处于应用域内;计算新化合物的期望核密度离群因子EKDOF(xp),若EKDOF(xp)小于训练集中化合物的EKDOF(xq)的最大值,则认为新化合物处于应用域内。EKDOF算法通过Python(v3.11.9,http://www.py-thon.org/)实现。应用构建的QSPR模型对外部数据集中的新化合物进行预测,比较3种应用域定义方法对模型预测准确率的影响。
2 结果与分析 2.1 有机发光材料健康危害分类基于C & L清单收录的分类与标签信息,61种OLEMs具有接触危害性,占收录OLEMs总数的64.9%,其中引起皮肤毒害作用、皮肤刺激、皮肤过敏反应、严重眼睛损伤或眼睛刺激的OLEMs分别占收录OLEMs总数的11.7%、51.1%、5.3%、9.6%和50.0%(表 1);51种OLEMs具有吸入危害性,占收录OLEMs总数的54.3%,其中可能引起毒害作用、过敏或哮喘症状、呼吸困难或呼吸道刺激的OLEMs分别占收录OLEMs总数的13.8%、1.1%和44.7%;50种OLEMs同时具有接触危害性和吸入危害性,占收录OLEMs总数的53.2%。
| 危害编码Hazard classifications | 危害说明Hazard statement | 有机发光材料种类Number of OLEMs | 有机发光材料占比/% Percentage of OLEMs/% |
| H311 | Toxic in contact with skin | 3 | 3.2 |
| H312 | Harmful in contact with skin | 8 | 8.5 |
| H315 | Causes skin irritation | 48 | 51.1 |
| H317 | May cause an allergic skin reaction | 5 | 5.3 |
| H318 | Causes serious eye damage | 9 | 9.6 |
| H319 | Causes serious eye irritation | 47 | 50.0 |
| H331 | Toxic if inhaled | 3 | 3.2 |
| H332 | Harmful if inhaled | 10 | 10.6 |
| H334 | May cause allergy or asthma symptoms or breathing difficulties if inhaled | 1 | 1.1 |
| H335 | May cause respiratory irritation | 42 | 44.7 |
| Note: hazard classifications are coded according to the Globally Harmonized System of Classification and Labelling of Chemicals[18]. | |||
卡方检验表明,造成皮肤毒害作用或刺激及过敏反应的OLEMs可能造成严重的眼睛损伤或眼睛刺激(P < 0.01),吸入有毒的OLEMs可能引起呼吸道过敏(P < 0.05),该结果与Corvaro等[19]及Gad等[20]的研究一致,说明不同毒性效应之间具有相关性。因此,本研究将与皮肤接触有毒害或刺激作用、可能造成严重眼睛损伤或刺激的OLEMs归为一类;将吸入有毒害作用、可能引起呼吸道过敏或刺激反应的OLEMs归为一类,分别构建接触危害性预测模型和吸入危害性预测模型。
2.2 有机发光材料定量构效关系模型如公式(6)及公式(7)所示,定义Tc为接触危害性预测概率值,Tb为吸入危害性预测概率值,若Tc或Tb ≥0.5,则模型预测该OLEM具有接触危害性或吸入危害性;若Tc或Tb < 0.5,则模型预测该OLEM不具有接触危害性或吸入危害性。变量含义如表 2所示。
| $ {T_{\rm{c}}} = 0.7889 + 0.8678 \cdot AATS5v{\cdot^3}\sqrt {GGI5} - 13.3461{\cdot^3}\sqrt {MATS2s} {\cdot^3}\sqrt {ATS2m} {\rm{ }} + 10.3975{\cdot^3}\sqrt {MATS2s} {\cdot^3}\sqrt {ATS1m} + 1.5793{\cdot^3}\sqrt {GGI5} {\cdot^3}\sqrt {AATS3V} - 1.9585 \cdot AATS3v{\cdot^3}\sqrt {ATS7e} + 1.7771 \cdot GGI3{\cdot^3}\sqrt {MATS2s} $ | (6) |
| $ {T_{\rm{b}}} = 0.3640 + 0.6375 \cdot ATS8p - 1.011 \cdot GATS7i + 0.3952 \cdot AATS3v + 0.3617 \cdot ATSC3i $ | (7) |
| 输入变量Input | 定义Definition | 参考文献Reference |
| ATS1m | Broto-Moreau autocorrelation-lag 1/weighted by mass | [21] |
| ATS2m | Broto-Moreau autocorrelation-lag 2/weighted by mass | [22] |
| GGI3 | Topological charge index of order 3 | [23] |
| GGI5 | Topological charge index of order 5 | [24] |
| ATS7e | Broto-Moreau autocorrelation-lag 7/weighted by Sanderson electronegativities | [25] |
| AATS5v | Average Broto-Moreau autocorrelation-lag 5/weighted by van der Waals volumes | [26] |
| MATS2s | Moran autocorrelation-lag 2/weighted by I-state | [27] |
| ATS8p | Broto-Moreau autocorrelation-lag 8/weighted by polarizabilities | [28] |
| GATS7i | Geary autocorrelation-lag 7/weighted by first ionization potential | [29] |
| ATSC3i | Centered Broto-Moreau autocorrelation-lag 3/weighted by first ionization potential | [30] |
| AATS3v | Average Broto-Moreau autocorrelation-lag 3/weighted by van der Waals volumes | [31] |
如表 3所示,新构建的QSPR模型对OLEMs的接触危害性的预测准确率、敏感性、阳性预测值和阴性预测值均高于STopTox、VEGA和QSAR Toolbox模型,仅在特异性方面略低于QSAR Toolbox模型,然而QSAR Toolbox模型较高的特异性是以牺牲敏感性为代价的,该模型并不能准确识别具有接触危害性的化合物。
| Unit: % | |||||||||||||||||||||||||||||
| 模型Models | 准确率ACC | 敏感性SEN | 特异性SPE | 阳性预测值PPV | 阴性预测值NPV | ||||||||||||||||||||||||
| STopTox | 58.0 | 87.3 | 9.1 | 61.5 | 30.0 | ||||||||||||||||||||||||
| VEGA | 57.0 | 80.0 | 15.2 | 63.2 | 29.4 | ||||||||||||||||||||||||
| QSAR Toolbox | 45.3 | 24.5 | 78.8 | 65.0 | 39.4 | ||||||||||||||||||||||||
| QSPR | 82.1 | 88.9 | 70.0 | 84.2 | 77.8 | ||||||||||||||||||||||||
如表 4所示,新构建的QSPR模型对OLEMs的吸入危害性的预测准确率、特异性、阳性预测值和阴性预测值均高于STopTox和RespiraTox模型,仅在敏感性方面低于RespiraTox模型,然而,RespiraTox模型较高的敏感性是以牺牲特异性为代价的,该模型不能准确识别无吸入危害性的化合物。综上,相较于现有模型,本研究新构建的QSPR模型能够较准确地预测OLEMs的接触和吸入危害性。
| Unit: % | |||||||||||||||||||||||||||||
| 模型Models | 准确率ACC | 敏感性SEN | 特异性SPE | 阳性预测值PPV | 阴性预测值NPV | ||||||||||||||||||||||||
| STopTox | 61.4 | 64.4 | 58.1 | 61.7 | 61.0 | ||||||||||||||||||||||||
| RespiraTox | 55.6 | 96.8 | 0.0 | 56.6 | 0.0 | ||||||||||||||||||||||||
| QSPR | 82.1 | 80.0 | 84.6 | 85.7 | 78.6 | ||||||||||||||||||||||||
2.3 定量构效关系模型的应用域
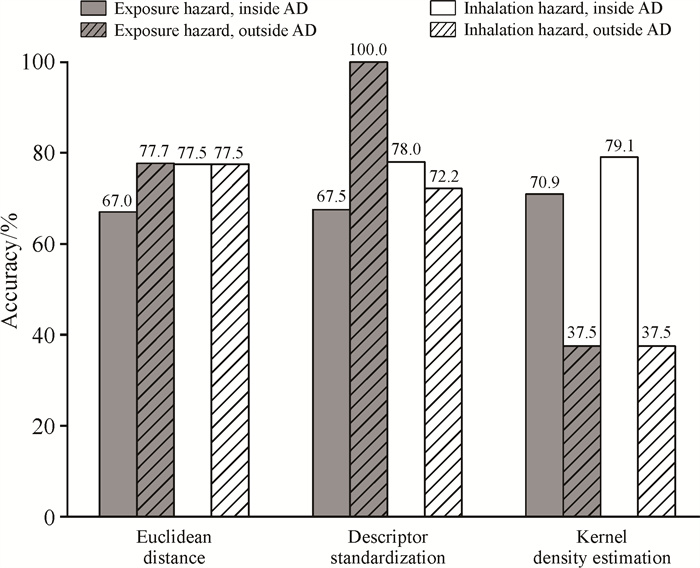
分别应用3种方法定义QSPR模型的应用域,预测外部数据集中类OLEMs分子的接触危害性及吸入危害性,结果如图 2所示。基于欧氏距离定义应用域时,接触危害性模型的预测准确率为67.0%-77.7%,吸入危害性模型对应用域内外的类OLEMs分子预测准确率均为77.5%;基于分子描述符标准化定义应用域时,接触危害性模型对应用域内外的类OLEMs分子预测准确率为67.5%-100.0%,吸入危害性模型的预测准确率为72.2%-78.0%;基于核密度估计的方法定义应用域时,接触危害性模型对应用域内外的类OLEMs分子预测准确率为37.5%-70.9%,吸入危害性模型的预测准确率为37.5%-79.1%。
|
| 图 2 3种方法在定量构效关系模型应用域内外预测准确率比较 Fig. 2 Comparison of prediction accuracy inside and outside the applicability domain of the quantitative structure-property relationship model among three methods |
基于欧氏距离及分子描述符标准化两种方法定义应用域时,QSPR模型对应用域内外化合物的预测准确率接近;基于分子描述符标准化定义应用域时,接触危害性模型对应用域外的化合物预测准确率甚至高于应用域内的预测准确率,说明两种方法不能有效定义QSPR模型的应用域[16]; 基于核密度估计的方法定义应用域时,QSPR模型对应用域内化合物的预测准确率明显高于对应用域外化合物的预测准确率,说明基于核密度估计的方法能够有效区分模型的应用域。
因此,本研究利用核密度估计的方法定义QSPR模型的应用域。在C & L清单未收录的1 894种OLEMs中,7种OLEMs处于接触危害性模型的应用域外,161种OLEMs处于吸入危害性模型的应用域外。对应用域内OLEMs的人体健康危害性进行预测,发现1 652种OLEMs可能具有接触危害性,占C & L清单未收录OLEMs总数的87.2%;1 612种OLEMs可能具有吸入危害性,占C & L清单未收录OLEMs总数的85.1%;1 428种OLEMs被认为同时具有接触和吸入危害性,占C & L清单未收录OLEMs总数的75.4%。
3 结论基于C & L清单收录的OLEMs人体健康危害分类与标签信息,本研究建立OLEMs接触、吸入危害性预测的QSPR模型并利用核密度估计定义模型的应用域,对OLEMs接触、吸入危害性的预测准确率均为82.1%,对应用域内类OLEMs分子的接触、吸入危害性预测准确率分别为70.9%、79.1%。预测结果显示,1 652种OLEMs可能引起皮肤刺激、严重眼睛损伤、皮肤过敏反应或眼睛刺激等接触危害性,1 612种OLEMs可能引起哮喘、呼吸困难或呼吸道刺激等吸入危害性。本研究构建的定量构效关系模型可用来快速预测评估OLEMs的健康危害,改善当前欧盟和美国国家环境保护局等机构限制动物实验而导致OLEMs健康危害特征信息收集缓慢的现状,进一步为优先控制化学品的筛选提供理论依据。
| [1] |
LI P T, YANG R, MA Y P, et al. Anthracene-triphenylamine materials with high luminance and negligible efficiency roll-off for organic light-emitting diodes[J]. Dyes and Pigments, 2024, 224: 112041. DOI:10.1016/j.dyepig.2024.112041 |
| [2] |
SATHIYAN G, BALASUBRAMANIAM B, RANJAN S, et al. A novel star-shaped triazine-triphenylamine-based fluorescent chemosensor for the selective detection of picric acid[J]. Materials Today Chemistry, 2019, 12: 178-186. DOI:10.1016/j.mtchem.2019.01.002 |
| [3] |
RAVI S, RAMAN T, PANNIPARA M, et al. Highly efficient red emissive zwitterionic fluorophores across wide pH range: white light emission, mechanofluorochromism, sensing chlorinated solvent and bioimaging[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2024, 453: 115655. DOI:10.1016/j.jphotochem.2024.115655 |
| [4] |
DUAN L, QIAO J, SUN Y D, et al. Strategies to design bipolar small molecules for OLEDs: donor-acceptor structure and non-donor-acceptor structure[J]. Advanced Materials, 2011, 23(9): 1137-1144. DOI:10.1002/adma.201003816 |
| [5] |
ZHU M S, SHEN M J, LIANG X X, et al. Identification of environmental liquid-crystal monomers: a class of new persistent organic pollutants-fluorinated biphenyls and analogues-emitted from E-waste dismantling[J]. Environmental Science & Technology, 2021, 55(9): 5984-5992. |
| [6] |
SHEN M J, FENG Z Q, LIANG X X, et al. Release and gas-particle partitioning behavior of liquid crystal monomers during the dismantling of waste liquid crystal display panels in E-waste recycling facilities[J]. Environmental Science & Technology, 2022, 56(5): 3106-3116. |
| [7] |
TAO D Y, JIN Q Q, RUAN Y F, et al. Widespread occurrence of emerging E-waste contaminants-liquid crystal monomers in sediments of the Pearl River Estuary, China[J]. Journal of Hazardous Materials, 2022, 437: 129377. DOI:10.1016/j.jhazmat.2022.129377 |
| [8] |
WANG J S, NAN J N, LI M, et al. First evidence of contamination in aquatic organisms with organic light-emitting materials[J]. Environmental Science & Technology Letters, 2022, 9(9): 739-746. |
| [9] |
ZHENG S P, WANG J S, LUO K, et al. Comprehensive characterization of organic light-emitting materials in breast milk by target and suspect screening[J]. Environmental Science & Technology, 2024, 58(11): 5103-5116. |
| [10] |
European Chemicals Agency. Guidance on registra- tion[R]. Helsinki: European Chemicals Agency, 2017.
|
| [11] |
European Chemicals Agency. Introductory guidance on the CLP regulation[R]. Helsinki: European Chemicals Agency, 2016.
|
| [12] |
FENG J J, SUN X F, ZENG E Y. Predicted health and environmental hazards of liquid crystal materials via quantitative structure-property relationship modeling[J]. Journal of Hazardous Materials, 2023, 446: 130592. DOI:10.1016/j.jhazmat.2022.130592 |
| [13] |
European Chemicals Agency. Guidance on information requirements and chemical safety assessment Chapter R. 6: QSARs and grouping of chemicals[R]. Helsinki: European Chemicals Agency, 2008.
|
| [14] |
JAWORSKA J, NIKOLOVA-JELIAZKOVA N, ALDENBERG T. QSAR applicability domain estimation by projection of the training set descriptor space: a review[J]. Alternatives to Laboratory Animals, 2005, 33(5): 445-459. DOI:10.1177/026119290503300508 |
| [15] |
NETZEVA T I, WORTH A, ALDENBERG T, et al. Current status of methods for defining the applicability domain of (quantitative) structure-activity relationships.The report and recommendations of ECVAM Workshop 52[J]. Alternatives to Laboratory Animals, 2005, 33(2): 155-173. DOI:10.1177/026119290503300209 |
| [16] |
ROY K, KAR S, AMBURE P. On a simple approach for determining applicability domain of QSAR models[J]. Chemometrics and Intelligent Laboratory Systems, 2015, 145: 22-29. DOI:10.1016/j.chemolab.2015.04.013 |
| [17] |
张忠平, 孙光旭, 姚春辰, 等. 基于期望核密度离群因子的离群点检测算法[J]. 高技术通讯, 2024, 34(2): 187-198. |
| [18] |
United Nations. Globally harmonized system of classification and labeling of chemicals[S]. New York and Geneva: United Nations, 2023: 282-285.
|
| [19] |
CORVARO M, GEHEN S, ANDREWS K, et al. A retrospective analysis of in vivo eye irritation, skin irritation and skin sensitisation studies with agrochemical formulations: setting the scene for development of alternative strategies[J]. Regulatory Toxicology and Pharmacology, 2017, 89: 131-147. |
| [20] |
GAD S C, WALSH R D, DUNN B J. Correlation of ocular and dermal irritancy of industrial chemicals[J]. Journal of Toxicology: Cutaneous and Ocular Toxicology, 1986, 5(3): 195-213. |
| [21] |
SIKORSKA C. Toward predicting vertical detachment energies for superhalogen anions exclusively from 2-D structures[J]. Chemical Physics Letters, 2015, 625: 157-163. |
| [22] |
REZIC T, VRSALOVIC PRESEC KI A, KURTANJEK & #381;. New approach to the evaluation of lignocellulose derived by-products impact on lytic-polysaccharide monooxygenase activity by using molecular descriptor structural causality model[J]. Bioresource Technology, 2021, 342: 125990. |
| [23] |
SHARMA B, PILANIA P, SINGH P. QSAR study on 5-lipoxygenase activating protein (FLAP) inhibitors: the derivatives of 2, 2-bisaryl-bicycloheptane[J]. Letters in Drug Design & Discovery, 2011, 8(1): 32-43. |
| [24] |
BERTATO L, CHIRICO N, PAPA E. QSAR models for the prediction of dietary biomagnification factor in fish[J]. Toxics, 2023, 11(3): 209. |
| [25] |
PRAJAPATI L M, PATEL J R, PARMAR V K. Descriptors requirement for QSAR analysis of pyrazolo-triazolo-pyrimidine derivative as human A3 receptor antagonists: design of novel furan derivatives and validation by docking[J]. Medicinal Chemistry Research, 2013, 23(5): 2554-2563. |
| [26] |
ALTAF R, NADEEM H, IQBAL M N, et al. Synthesis, biological evaluation, 2D-QSAR, and molecular simulation studies of dihydropyrimidinone derivatives as alkaline phosphatase inhibitors[J]. ACS Omega, 2022, 7(8): 7139-7154. |
| [27] |
MALI S N, PANDEY A, THORAT B R, et al. Multiple 3D- and 2D-quantitative structure-activity relationship models (QSAR), theoretical study and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against tuberculosis[J]. Structural Chemistry, 2022, 33(3): 679-694. |
| [28] |
AHMADINEJAD N, SHAFIEI F, ISFAHANI T M. Quantitative structure-property relationship (QSPR) investigation of camptothecin drugs derivatives[J]. Combinatorial Chemistry & High Throughput Screening, 2018, 21(7): 533-542. |
| [29] |
ARTHUR D E, UZAIRU A, MAMZA P, et al. Quantum modelling of the structure-activity and toxicity relationship studies of some potent compounds on SR leukemia cell line[J]. Chemical Data Collections, 2016, 5: 46-61. |
| [30] |
IBRAHIM Z Y, UZAIRU A, SHALLANGWA G, et al. Theoretical design of novel antimalarial agents against P.falciparum strain, Dd2 through the QSAR modeling of synthesized 2'-substituted triclosan derivatives[J]. Heliyon, 2020, 6(9): e05032. |
| [31] |
YU J H, RUAN S S, SONG H W, et al. Machine learning rate constants of hydrogen abstraction reactions between ester and H atom[J]. Combustion and Flame, 2023, 255: 112901. |



